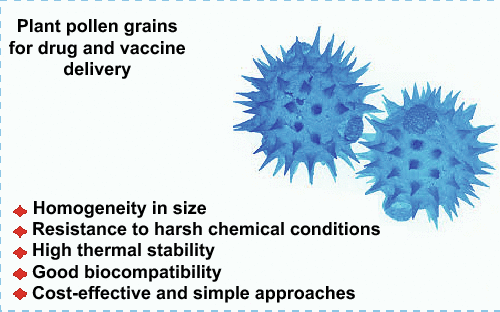
Plant Pollen Grains: A Move Towards Green Drug and Vaccine Delivery Systems
Corresponding Author: Rajender S. Varma
Nano-Micro Letters,
Vol. 13 (2021), Article Number: 128
Abstract
Pollen grains and plant spores have emerged as innovative biomaterials for various applications such as drug/vaccine delivery, catalyst support, and the removal of heavy metals. The natural microcapsules comprising spore shells and pollen grain are designed for protecting the genetic materials of plants from exterior impairments. Two layers make up the shell, the outer layer (exine) that comprised largely of sporopollenin, and the inner layer (intine) that built chiefly of cellulose. These microcapsule shells, namely hollow sporopollenin exine capsules have some salient features such as homogeneity in size, non-toxic nature, resilience to both alkalis and acids, and the potential to withstand at elevated temperatures; they have displayed promising potential for the microencapsulation and the controlled drug delivery/release. The important attribute of mucoadhesion to intestinal tissues can prolong the interaction of sporopollenin with the intestinal mucosa directing to an augmented effectiveness of nutraceutical or drug delivery. Here, current trends and prospects related to the application of plant pollen grains for the delivery of vaccines and drugs and vaccine are discussed.
Highlights:
1 Plant pollen grains and plant spores have emerged as innovative biomaterials for various applications.
2 Current trends and prospects related to the application of plant pollen grains for the delivery of vaccines and drugs are discussed.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- S. Krol, R. Macrez, F. Docagne, G. Defer, S. Laurent et al., Therapeutic benefits from nanops: the potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem. Rev. 113, 1877–1903 (2012). https://doi.org/10.1021/cr200472g
- P.-L. Lam, W.-Y. Wong, Z. Bian, C.-H. Chui, R. Gambari, Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomedicine 12, 357–385 (2017). https://doi.org/10.2217/nnm-2016-0305
- R. Mo, T. Jiang, Z. Gu, Recent progress in multidrug delivery to cancer cells by liposomes. Nanomedicine 9, 1117–1120 (2014). https://doi.org/10.2217/nnm.14.62
- M. Nasrollahzadeh, S.M. Sajadi, M. Sajjadi, Z. Issaabadi, An Introduction to Nanotechnology, in: Interface Science and Technology, Elsevier, 2019, pp. 1–27. https://doi.org/10.1016/B978-0-12-813586-0.00001-8
- T. Denk, M.V. Tekleva, Comparative pollen morphology and ultrastructure of Platanus: implications for phylogeny and evaluation of the fossil record. Grana 45, 195–221 (2006). https://doi.org/10.1080/00173130600873901
- S.A. Hamad, A.F.K. Dyab, S.D. Stoyanov, V.N. Paunov, Encapsulation of living cells into sporopollenin microcapsules. J. Mater. Chem. 21, 18018–18023 (2011). https://doi.org/10.1039/c1jm13719k
- A. Diego-Taboada, L. Maillet, J.H. Banoub, M. Lorch, A.S. Rigby et al., Protein free microcapsules obtained from plant spores as a model for drug delivery: ibuprofen encapsulation, release and taste masking. J. Mater. Chem. B 1, 707–713 (2013). https://doi.org/10.1039/C2TB00228K
- I. Palazzo, A. Mezzetta, L. Guazzelli, S. Sartini, C.S. Pomelli et al., Chiral ionic liquids supported on natural sporopollenin microcapsules. RSC Adv. 8, 21174–21183 (2018). https://doi.org/10.1039/C8RA03455A
- M.J. Uddin, H.S. Gill, From allergen to oral vaccine carrier: a new face of ragweed pollen. Int. J. Pharmac. 545, 286–294 (2018). https://doi.org/10.1016/j.ijpharm.2018.05.003
- C.S. Pomelli, F. D’Andrea, A. Mezzetta, L. Guazzelli, Exploiting pollen and sporopollenin for thesustainable production of microstructures. New J. Chem. 44, 647–652 (2020). https://doi.org/10.1039/C9NJ05082E
- J.H. Park, J. Seo, J.A. Jackman, N.-J. Cho, Inflated sporopollenin exine capsules obtained from thin-walled pollen. Sci. Rep. 6, 28017 (2016). https://doi.org/10.1038/srep28017
- S.L. Atkin, S. Barrier, Z. Cui, P.D. Fletcher, G. Mackenzie et al., UV and visible light screening by individual sporopollenin exines derived from Lycopodium clavatum (club moss) and Ambrosia trifida (giant ragweed). J. Photochem. Photobiol. B Biol. 102, 209–217 (2011). https://doi.org/10.1016/j.jphotobiol.2010.12.005
- A. Diego-Taboada, T.S. Beckett, L.S. Atkin, G. Mackenzie, Hollow pollen shells to enhance drug delivery. Pharmaceutics 6, 80–96 (2014). https://doi.org/10.3390/pharmaceutics6010080
- B. Luppi, T. Cerchiara, F. Bigucci, I. Orienti, V. Zecchi, pH-sensitive polymeric physical-mixture for possible site-specific delivery of ibuprofen. Eur. J. Pharm. Biopharm. 55, 199–202 (2003). https://doi.org/10.1016/S0939-6411(02)00190-X
- V.N. Paunov, G. Mackenzie, S.D. Stoyanov, Sporopollenin micro-reactors for in-situ preparation, encapsulation and targeted delivery of active components. J. Mater. Chem. 17, 609–612 (2007). https://doi.org/10.1039/b615865j
- S. Barrier, A. Diego-Taboada, M.J. Thomasson, L. Madden, J.C. Pointon et al., Viability of plant spore exine capsules for microencapsulation. J. Mater. Chem. 21, 975–981 (2011). https://doi.org/10.1039/C0JM02246B
- W. Brandon Goodwin, I.J. Gomez, Y. Fang, J.C. Meredith, K.H. Sandhage, Conversion of pollen ps into three-dimensional ceramic replicas tailored for multimodal adhesion. Chem. Mater. 25, 4529–4536 (2013). https://doi.org/10.1021/cm402226w
- M.J. Uddin, H.S. Gill, Ragweed pollen as an oral vaccine delivery system: mechanistic insights. J. Control. Release 268, 416–426 (2017). https://doi.org/10.1016/j.jconrel.2017.10.019
- S.M. Alshehri, H.A. Al-Lohedan, E. Al-Farraj, N. Alhokbany, A.A. Chaudhary et al., Macroporous natural capsules extracted from Phoenix dactylifera L. spore and their application in oral drugs delivery. Int. J. Pharm. 504, 39–47 (2016). https://doi.org/10.1016/j.ijpharm.2016.02.049
- S.M. Alshehri, H.A. Al-Lohedan, A.A. Chaudhary, E. Al-Farraj, N. Alhokbany et al., Delivery of ibuprofen by natural macroporous sporopollenin exine capsules extracted from Phoenix dactylifera L. Eur. J. Pharm. Sci. 88, 158–165 (2016). https://doi.org/10.1016/j.ejps.2016.02.004
- T.L. Harris, C.J. Wenthur, A. Diego-Taboada, G. Mackenzie, T.S. Corbitt et al., Lycopodium clavatum exine microcapsules enable safe oral delivery of 3,4-diaminopyridine for treatment of botulinum neurotoxin A intoxication. Chem. Commun. 52, 4187–4190 (2016). https://doi.org/10.1039/C6CC00615A
- R.C. Mundargi, M.G. Potroz, J.H. Park, J. Seo, J.H. Lee et al., Extraction of sporopollenin exine capsules from sunflower pollen grains. RSC Adv. 6, 16533–16539 (2016). https://doi.org/10.1039/C5RA27207F
- R.C. Mundargi, M.G. Potroz, S. Park, J.H. Park, H. Shirahama et al., Lycopodium spores: a naturally manufactured, superrobust biomaterial for drug delivery. Adv. Funct. Mater. 26, 487–497 (2016). https://doi.org/10.1002/adfm.201502322
- S.V. Lale, H.S. Gill, Pollen grains as a novel microcarrier for oral delivery of proteins. Int. J. Pharm. 552, 352–359 (2018). https://doi.org/10.1016/j.ijpharm.2018.10.016
- D. Wu, X. Wang, S. Wang, B. Li, H. Liang, Nanop encapsulation strategy: leveraging plant exine capsules used as secondary capping for oral delivery. J. Agric. Food Chem. 67, 8168–8176 (2019). https://doi.org/10.1021/acs.jafc.9b02003
- W.J. Guilford, D.M. Schneider, J. Labovitz, S.J. Opella, High resolution solid state (13)c nmr spectroscopy of sporopollenins from different plant taxa. Plant Physiol. 86, 134–136 (1988). https://doi.org/10.1104/pp.86.1.134
- G. Shaw, A. Yeadon, Chemical studies on the constitution of some pollen and spore membranes. J. Chem. Soci. C Org. 16–22 (1966). https://doi.org/10.1039/j39660000016
- G. Shaw, A. Yeadon, Chemical studies on the constitution of some pollen and spore membranes. Grana Palynol. 5, 247–252 (1964). https://doi.org/10.1080/00173136409430017
- Y. Xu, N. Shrestha, V. Préat, A. Beloqui, Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. J. Control. Release 322, 486–508 (2020). https://doi.org/10.1016/j.jconrel.2020.04.006
- C. Jungfermann, F. Ahlers, M. Grote, S. Gubatz, S. Steuernagel et al., Solution of sporopollenin and reaggregation of a sporopollenin-like material: a new approach in the sporopollenin research. J. Plant Physiol 151, 513–519 (1997). https://doi.org/10.1016/S0176-1617(97)80224-6
- A.V.V. Nikezić, A.M. Bondžić, V.M. Vasić, Drug delivery systems based on nanops and related nanostructures. Eur. J. Pharm. Sci. 151, 105412 (2020). https://doi.org/10.1016/j.ejps.2020.105412
- S. Park, H. Chin, Y. Hwang, T.-F. Fan, N.-J. Cho, A facile approach to patterning pollen microps for in situ imaging. Appl. Mater. Today 20, 100702 (2020). https://doi.org/10.1016/j.apmt.2020.100702
- P. Gonzalez-Cruz, M.J. Uddin, S.U. Atwe, N. Abidi, H.S. Gill, A chemical treatment method for obtaining clean and intact pollen shells of different species. ACS Biomater. Sci. Eng. 4, 2319–2329 (2018). https://doi.org/10.1021/acsbiomaterials.8b00304
- T. Maric, M.Z. Mohamad Nasir, N.F. Rosli, M. Budanović, R.D. Webster et al., Microrobots derived from variety plant pollen grains for efficient environmental clean up and as an anti‐cancer drug carrier. Adv. Funct. Mater. 30, 2000112 (2020). https://doi.org/10.1002/adfm.202000112
- D. Wu, Y. Liang, Y. Pei, B. Li, H. Liang, Plant exine capsules based encapsulation strategy: a high loading and long-term effective delivery system for nobiletin. Food Res. Int. 127, 108691 (2020). https://doi.org/10.1016/j.foodres.2019.108691
- T. Fan, J.H. Park, Q.A. Pham, E.-L. Tan, R.C. Mundargi et al., Extraction of cage-like sporopollenin exine capsules from dandelion pollen grains. Sci. Rep. 8, 6565 (2018). https://doi.org/10.1038/s41598-018-24336-9
- Z. Deng, Y. Pei, S. Wang, B. Zhou, X. Hou et al., Designable carboxymethylpachymaran/metal ion architecture on sunflower sporopollenin exine capsules as delivery vehicles for bioactive macromolecules. J. Agric. Food Chem. 68, 13990–14000 (2020). https://doi.org/10.1021/acs.jafc.0c05169
- M. Mujtaba, I. Sargin, L. Akyuz, T. Ceter, M. Kaya, Newly isolated sporopollenin microcages from Platanus orientalis pollens as a vehicle for controlled drug delivery. Mater. Sci. Eng. C 77, 263–270 (2017). https://doi.org/10.1016/j.msec.2017.02.176
- M. Mujtaba, B.A. Yılmaz, D. Cansaran-Duman, L. Akyuz, S. Yangın et al., Newly isolated sporopollenin microcages from Cedrus libani and Pinus nigra for controlled delivery of Oxaliplatin. bioRxiv (2020). https://doi.org/10.1101/2020.10.19.345157
- I. Sargin, L. Akyuz, M. Kaya, G. Tan, T. Ceter et al., Controlled release and anti-proliferative effect of imatinib mesylate loaded sporopollenin microcapsules extracted from pollens of Betula pendula. Int. J. Biol. Macromol. 105, 749–756 (2017). https://doi.org/10.1016/j.ijbiomac.2017.07.093
- A.K.F. Dyab, M.A. Mohamed, N.M. Meligi, S.K. Mohamed, Encapsulation of erythromycin and bacitracin antibiotics into natural sporopollenin microcapsules: antibacterial, cytotoxicity, in vitro and in vivo release studies for enhanced bioavailability. RSC Adv. 8, 33432–33444 (2018). https://doi.org/10.1039/C8RA05499A
- C.S. Bailey, J.S. Zarins-Tutt, M. Agbo, H. Gao, A. Diego-Taboada et al., A natural solution to photoprotection and isolation of the potent polyene antibiotic, marinomycin A. Chem. Sci. 10, 7549–7553 (2019). https://doi.org/10.1039/C9SC01375J
- M.J. Thomasson, A. Diego-Taboada, S. Barrier, J. Martin-Guyout, E. Amedjou et al., Sporopollenin exine capsules (SpECs) derived from Lycopodium clavatum provide practical antioxidant properties by retarding rancidification of an ω-3 oil. Ind. Crops. Prod. 154, 112714 (2020). https://doi.org/10.1016/j.indcrop.2020.112714
- M.K. Corliss, C.K. Bok, J. Gillissen, M.G. Potroz, H. Jung et al., Preserving the inflated structure of lyophilized sporopollenin exine capsules with polyethylene glycol osmolyte. J. Ind. Eng. Chem. 61, 255–264 (2018). https://doi.org/10.1016/j.jiec.2017.12.023
- L. Akyuz, I. Sargin, M. Kaya, T. Ceter, I. Akata, A new pollen-derived microcarrier for pantoprazole delivery. Mater. Sci. Eng. C 71, 937–942 (2017). https://doi.org/10.1016/j.msec.2016.11.009
- S.U. Atwe, Y. Ma, H.S. Gill, Pollen grains for oral vaccination. J. Control. Release 194, 45–52 (2014). https://doi.org/10.1016/j.jconrel.2014.08.010
- H.S. Gill, Transforming pollen grains from an allergy causing material into a biomaterial for oral vaccination. Southwest Respir. Crit. Care Chron. 7, 4–6 (2019). https://doi.org/10.12746/swrccc.v7i27.510
References
S. Krol, R. Macrez, F. Docagne, G. Defer, S. Laurent et al., Therapeutic benefits from nanops: the potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem. Rev. 113, 1877–1903 (2012). https://doi.org/10.1021/cr200472g
P.-L. Lam, W.-Y. Wong, Z. Bian, C.-H. Chui, R. Gambari, Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomedicine 12, 357–385 (2017). https://doi.org/10.2217/nnm-2016-0305
R. Mo, T. Jiang, Z. Gu, Recent progress in multidrug delivery to cancer cells by liposomes. Nanomedicine 9, 1117–1120 (2014). https://doi.org/10.2217/nnm.14.62
M. Nasrollahzadeh, S.M. Sajadi, M. Sajjadi, Z. Issaabadi, An Introduction to Nanotechnology, in: Interface Science and Technology, Elsevier, 2019, pp. 1–27. https://doi.org/10.1016/B978-0-12-813586-0.00001-8
T. Denk, M.V. Tekleva, Comparative pollen morphology and ultrastructure of Platanus: implications for phylogeny and evaluation of the fossil record. Grana 45, 195–221 (2006). https://doi.org/10.1080/00173130600873901
S.A. Hamad, A.F.K. Dyab, S.D. Stoyanov, V.N. Paunov, Encapsulation of living cells into sporopollenin microcapsules. J. Mater. Chem. 21, 18018–18023 (2011). https://doi.org/10.1039/c1jm13719k
A. Diego-Taboada, L. Maillet, J.H. Banoub, M. Lorch, A.S. Rigby et al., Protein free microcapsules obtained from plant spores as a model for drug delivery: ibuprofen encapsulation, release and taste masking. J. Mater. Chem. B 1, 707–713 (2013). https://doi.org/10.1039/C2TB00228K
I. Palazzo, A. Mezzetta, L. Guazzelli, S. Sartini, C.S. Pomelli et al., Chiral ionic liquids supported on natural sporopollenin microcapsules. RSC Adv. 8, 21174–21183 (2018). https://doi.org/10.1039/C8RA03455A
M.J. Uddin, H.S. Gill, From allergen to oral vaccine carrier: a new face of ragweed pollen. Int. J. Pharmac. 545, 286–294 (2018). https://doi.org/10.1016/j.ijpharm.2018.05.003
C.S. Pomelli, F. D’Andrea, A. Mezzetta, L. Guazzelli, Exploiting pollen and sporopollenin for thesustainable production of microstructures. New J. Chem. 44, 647–652 (2020). https://doi.org/10.1039/C9NJ05082E
J.H. Park, J. Seo, J.A. Jackman, N.-J. Cho, Inflated sporopollenin exine capsules obtained from thin-walled pollen. Sci. Rep. 6, 28017 (2016). https://doi.org/10.1038/srep28017
S.L. Atkin, S. Barrier, Z. Cui, P.D. Fletcher, G. Mackenzie et al., UV and visible light screening by individual sporopollenin exines derived from Lycopodium clavatum (club moss) and Ambrosia trifida (giant ragweed). J. Photochem. Photobiol. B Biol. 102, 209–217 (2011). https://doi.org/10.1016/j.jphotobiol.2010.12.005
A. Diego-Taboada, T.S. Beckett, L.S. Atkin, G. Mackenzie, Hollow pollen shells to enhance drug delivery. Pharmaceutics 6, 80–96 (2014). https://doi.org/10.3390/pharmaceutics6010080
B. Luppi, T. Cerchiara, F. Bigucci, I. Orienti, V. Zecchi, pH-sensitive polymeric physical-mixture for possible site-specific delivery of ibuprofen. Eur. J. Pharm. Biopharm. 55, 199–202 (2003). https://doi.org/10.1016/S0939-6411(02)00190-X
V.N. Paunov, G. Mackenzie, S.D. Stoyanov, Sporopollenin micro-reactors for in-situ preparation, encapsulation and targeted delivery of active components. J. Mater. Chem. 17, 609–612 (2007). https://doi.org/10.1039/b615865j
S. Barrier, A. Diego-Taboada, M.J. Thomasson, L. Madden, J.C. Pointon et al., Viability of plant spore exine capsules for microencapsulation. J. Mater. Chem. 21, 975–981 (2011). https://doi.org/10.1039/C0JM02246B
W. Brandon Goodwin, I.J. Gomez, Y. Fang, J.C. Meredith, K.H. Sandhage, Conversion of pollen ps into three-dimensional ceramic replicas tailored for multimodal adhesion. Chem. Mater. 25, 4529–4536 (2013). https://doi.org/10.1021/cm402226w
M.J. Uddin, H.S. Gill, Ragweed pollen as an oral vaccine delivery system: mechanistic insights. J. Control. Release 268, 416–426 (2017). https://doi.org/10.1016/j.jconrel.2017.10.019
S.M. Alshehri, H.A. Al-Lohedan, E. Al-Farraj, N. Alhokbany, A.A. Chaudhary et al., Macroporous natural capsules extracted from Phoenix dactylifera L. spore and their application in oral drugs delivery. Int. J. Pharm. 504, 39–47 (2016). https://doi.org/10.1016/j.ijpharm.2016.02.049
S.M. Alshehri, H.A. Al-Lohedan, A.A. Chaudhary, E. Al-Farraj, N. Alhokbany et al., Delivery of ibuprofen by natural macroporous sporopollenin exine capsules extracted from Phoenix dactylifera L. Eur. J. Pharm. Sci. 88, 158–165 (2016). https://doi.org/10.1016/j.ejps.2016.02.004
T.L. Harris, C.J. Wenthur, A. Diego-Taboada, G. Mackenzie, T.S. Corbitt et al., Lycopodium clavatum exine microcapsules enable safe oral delivery of 3,4-diaminopyridine for treatment of botulinum neurotoxin A intoxication. Chem. Commun. 52, 4187–4190 (2016). https://doi.org/10.1039/C6CC00615A
R.C. Mundargi, M.G. Potroz, J.H. Park, J. Seo, J.H. Lee et al., Extraction of sporopollenin exine capsules from sunflower pollen grains. RSC Adv. 6, 16533–16539 (2016). https://doi.org/10.1039/C5RA27207F
R.C. Mundargi, M.G. Potroz, S. Park, J.H. Park, H. Shirahama et al., Lycopodium spores: a naturally manufactured, superrobust biomaterial for drug delivery. Adv. Funct. Mater. 26, 487–497 (2016). https://doi.org/10.1002/adfm.201502322
S.V. Lale, H.S. Gill, Pollen grains as a novel microcarrier for oral delivery of proteins. Int. J. Pharm. 552, 352–359 (2018). https://doi.org/10.1016/j.ijpharm.2018.10.016
D. Wu, X. Wang, S. Wang, B. Li, H. Liang, Nanop encapsulation strategy: leveraging plant exine capsules used as secondary capping for oral delivery. J. Agric. Food Chem. 67, 8168–8176 (2019). https://doi.org/10.1021/acs.jafc.9b02003
W.J. Guilford, D.M. Schneider, J. Labovitz, S.J. Opella, High resolution solid state (13)c nmr spectroscopy of sporopollenins from different plant taxa. Plant Physiol. 86, 134–136 (1988). https://doi.org/10.1104/pp.86.1.134
G. Shaw, A. Yeadon, Chemical studies on the constitution of some pollen and spore membranes. J. Chem. Soci. C Org. 16–22 (1966). https://doi.org/10.1039/j39660000016
G. Shaw, A. Yeadon, Chemical studies on the constitution of some pollen and spore membranes. Grana Palynol. 5, 247–252 (1964). https://doi.org/10.1080/00173136409430017
Y. Xu, N. Shrestha, V. Préat, A. Beloqui, Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. J. Control. Release 322, 486–508 (2020). https://doi.org/10.1016/j.jconrel.2020.04.006
C. Jungfermann, F. Ahlers, M. Grote, S. Gubatz, S. Steuernagel et al., Solution of sporopollenin and reaggregation of a sporopollenin-like material: a new approach in the sporopollenin research. J. Plant Physiol 151, 513–519 (1997). https://doi.org/10.1016/S0176-1617(97)80224-6
A.V.V. Nikezić, A.M. Bondžić, V.M. Vasić, Drug delivery systems based on nanops and related nanostructures. Eur. J. Pharm. Sci. 151, 105412 (2020). https://doi.org/10.1016/j.ejps.2020.105412
S. Park, H. Chin, Y. Hwang, T.-F. Fan, N.-J. Cho, A facile approach to patterning pollen microps for in situ imaging. Appl. Mater. Today 20, 100702 (2020). https://doi.org/10.1016/j.apmt.2020.100702
P. Gonzalez-Cruz, M.J. Uddin, S.U. Atwe, N. Abidi, H.S. Gill, A chemical treatment method for obtaining clean and intact pollen shells of different species. ACS Biomater. Sci. Eng. 4, 2319–2329 (2018). https://doi.org/10.1021/acsbiomaterials.8b00304
T. Maric, M.Z. Mohamad Nasir, N.F. Rosli, M. Budanović, R.D. Webster et al., Microrobots derived from variety plant pollen grains for efficient environmental clean up and as an anti‐cancer drug carrier. Adv. Funct. Mater. 30, 2000112 (2020). https://doi.org/10.1002/adfm.202000112
D. Wu, Y. Liang, Y. Pei, B. Li, H. Liang, Plant exine capsules based encapsulation strategy: a high loading and long-term effective delivery system for nobiletin. Food Res. Int. 127, 108691 (2020). https://doi.org/10.1016/j.foodres.2019.108691
T. Fan, J.H. Park, Q.A. Pham, E.-L. Tan, R.C. Mundargi et al., Extraction of cage-like sporopollenin exine capsules from dandelion pollen grains. Sci. Rep. 8, 6565 (2018). https://doi.org/10.1038/s41598-018-24336-9
Z. Deng, Y. Pei, S. Wang, B. Zhou, X. Hou et al., Designable carboxymethylpachymaran/metal ion architecture on sunflower sporopollenin exine capsules as delivery vehicles for bioactive macromolecules. J. Agric. Food Chem. 68, 13990–14000 (2020). https://doi.org/10.1021/acs.jafc.0c05169
M. Mujtaba, I. Sargin, L. Akyuz, T. Ceter, M. Kaya, Newly isolated sporopollenin microcages from Platanus orientalis pollens as a vehicle for controlled drug delivery. Mater. Sci. Eng. C 77, 263–270 (2017). https://doi.org/10.1016/j.msec.2017.02.176
M. Mujtaba, B.A. Yılmaz, D. Cansaran-Duman, L. Akyuz, S. Yangın et al., Newly isolated sporopollenin microcages from Cedrus libani and Pinus nigra for controlled delivery of Oxaliplatin. bioRxiv (2020). https://doi.org/10.1101/2020.10.19.345157
I. Sargin, L. Akyuz, M. Kaya, G. Tan, T. Ceter et al., Controlled release and anti-proliferative effect of imatinib mesylate loaded sporopollenin microcapsules extracted from pollens of Betula pendula. Int. J. Biol. Macromol. 105, 749–756 (2017). https://doi.org/10.1016/j.ijbiomac.2017.07.093
A.K.F. Dyab, M.A. Mohamed, N.M. Meligi, S.K. Mohamed, Encapsulation of erythromycin and bacitracin antibiotics into natural sporopollenin microcapsules: antibacterial, cytotoxicity, in vitro and in vivo release studies for enhanced bioavailability. RSC Adv. 8, 33432–33444 (2018). https://doi.org/10.1039/C8RA05499A
C.S. Bailey, J.S. Zarins-Tutt, M. Agbo, H. Gao, A. Diego-Taboada et al., A natural solution to photoprotection and isolation of the potent polyene antibiotic, marinomycin A. Chem. Sci. 10, 7549–7553 (2019). https://doi.org/10.1039/C9SC01375J
M.J. Thomasson, A. Diego-Taboada, S. Barrier, J. Martin-Guyout, E. Amedjou et al., Sporopollenin exine capsules (SpECs) derived from Lycopodium clavatum provide practical antioxidant properties by retarding rancidification of an ω-3 oil. Ind. Crops. Prod. 154, 112714 (2020). https://doi.org/10.1016/j.indcrop.2020.112714
M.K. Corliss, C.K. Bok, J. Gillissen, M.G. Potroz, H. Jung et al., Preserving the inflated structure of lyophilized sporopollenin exine capsules with polyethylene glycol osmolyte. J. Ind. Eng. Chem. 61, 255–264 (2018). https://doi.org/10.1016/j.jiec.2017.12.023
L. Akyuz, I. Sargin, M. Kaya, T. Ceter, I. Akata, A new pollen-derived microcarrier for pantoprazole delivery. Mater. Sci. Eng. C 71, 937–942 (2017). https://doi.org/10.1016/j.msec.2016.11.009
S.U. Atwe, Y. Ma, H.S. Gill, Pollen grains for oral vaccination. J. Control. Release 194, 45–52 (2014). https://doi.org/10.1016/j.jconrel.2014.08.010
H.S. Gill, Transforming pollen grains from an allergy causing material into a biomaterial for oral vaccination. Southwest Respir. Crit. Care Chron. 7, 4–6 (2019). https://doi.org/10.12746/swrccc.v7i27.510