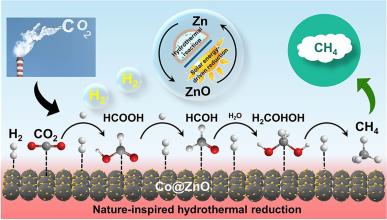
100% Conversion of CO2–CH4 with Non-Precious Co@ZnO Catalyst in Hot Water
Corresponding Author: Fangming Jin
Nano-Micro Letters,
Vol. 17 (2025), Article Number: 216
Abstract
The combination of solar energy and natural hydrothermal systems will innovate the chemistry of CO2 hydrogenation; however, the approach remains challenging due to the lack of robust and cost-effective catalytic system. Here, Zn which can be recycled with solar energy-induced approach was chosen as the reductant and Co as catalyst to achieve robust hydrothermal CO2 methanation. Nanosheets of honeycomb ZnO were grown in situ on the Co surface, resulting in a new motif (Co@ZnO catalyst) that inhibits Co deactivation through ZnO-assisted CoOx reduction. The stabilized Co and interaction between Co and ZnO functioned collaboratively toward the full conversion of CO2–CH4. In situ hydrothermal infrared spectroscopy confirmed the formation of formic acid as an intermediate, thereby avoiding CO formation and unwanted side reaction pathways. This study presents a straightforward one-step process for both highly efficient CO2 conversion and catalyst synthesis, paving the way for solar-driven CO2 methanation.
Highlights:
1 The combination of solar energy and underground hydrothermal environment supports the sustained and efficient CH4 production from CO2.
2 Nanosheets of honeycomb ZnO were formed in-situ on the Co surface, resulting in a new motif (Co@ZnO catalyst) that inhibits Co deactivation through ZnO-assisted CoOx reduction.
3 The stabilized Co and interaction between Co and ZnO inhibited unwanted side reaction pathways via CO production, ensuring formic acid formed as an intermediate, leading to 100% CH4 yield.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- P. Gai, W. Yu, H. Zhao, R. Qi, F. Li et al., Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid. Angew. Chem. Int. Ed. 59(18), 7224–7229 (2020). https://doi.org/10.1002/anie.202001047
- C. Ban, Y. Duan, Y. Wang, J. Ma, K. Wang et al., Isotype heterojunction-boosted CO2 photoreduction to CO. Nano-Micro Lett. 14(1), 74 (2022). https://doi.org/10.1007/s40820-022-00821-9
- U. Kang, S.K. Choi, D.J. Ham, S.M. Ji, W. Choi et al., Photosynthesis of formate from CO2 and water at 1% energy efficiency via copper iron oxide catalysis. Energy Environ. Sci. 8(9), 2638–2643 (2015). https://doi.org/10.1039/c5ee01410g
- K. Wang, Z. Hu, P. Yu, A.M. Balu, K. Li et al., Understanding bridging sites and accelerating quantum efficiency for photocatalytic CO2 reduction. Nano-Micro Lett. 16(1), 5 (2023). https://doi.org/10.1007/s40820-023-01221-3
- X. Meng, T. Wang, L. Liu, S. Ouyang, P. Li et al., Photothermal conversion of CO2 into CH4 with H2 over group VIII nanocatalysts: an alternative approach for solar fuel production. Angew. Chem. Int. Ed. 53, 11478–11482 (2014). https://doi.org/10.1002/anie.201404953
- W.C. Chueh, C. Falter, M. Abbott, D. Scipio, P. Furler et al., High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330(6012), 1797–1801 (2010). https://doi.org/10.1126/science.1197834
- A. Steinfeld, Solar thermochemical production of hydrogen–– a review. Sol. Energy 78(5), 603–615 (2005). https://doi.org/10.1016/j.solener.2003.12.012
- L.O. Schunk, A. Steinfeld, Kinetics of the thermal dissociation of ZnO exposed to concentrated solar irradiation using a solar-driven thermogravimeter in the 1800–2100 K range. AlChE J. 55, 1497–1504 (2009). https://doi.org/10.1002/aic.11765
- L.O. Schunk, W. Lipiński, A. Steinfeld, Heat transfer model of a solar receiver-reactor for the thermal dissociation of ZnO: experimental validation at 10 kW and scale-up to 1MW. Chem. Eng. J. 150(2–3), 502–508 (2009). https://doi.org/10.1016/j.cej.2009.03.012
- T. Kodama, N. Gokon, Thermochemical cycles for high-temperature solar hydrogen production. Chem. Rev. 107, 4048–4077 (2007). https://doi.org/10.1021/cr050188a
- M. Chambon, S. Abanades, G. Flamant, Solar thermal reduction of ZnO and SnO2: characterization of the recombination reaction with O2. Chem. Eng. Sci. 65(11), 3671–3680 (2010). https://doi.org/10.1016/j.ces.2010.03.005
- D. Gstoehl, A. Brambilla, L.O. Schunk, A. Steinfeld, A quenching apparatus for the gaseous products of the solar thermal dissociation of ZnO. J. Mater. Sci. 43, 4729–4736 (2008). https://doi.org/10.1007/s10853-007-2351-x
- B. Sherwood Lollar, T.D. Westgate, J.A. Ward, G.F. Slater, G. Lacrampe-Couloume, Abiogenic formation of alkanes in the earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 416(6880), 522–524 (2002). https://doi.org/10.1038/416522a
- Q. Fu, B. Sherwood Lollar, J. Horita, G. Lacrampe-Couloume, W.E. Seyfried, Abiotic formation of hydrocarbons under hydrothermal conditions: constraints from chemical and isotope data. Geochim. Cosmochim. Acta 71(8), 1982–1998 (2007). https://doi.org/10.1016/j.gca.2007.01.022
- J.M. McDermott, J.S. Seewald, C.R. German, S.P. Sylva, Pathways for abiotic organic synthesis at submarine hydrothermal fields. PNAS 112, 7668–7672 (2015). https://doi.org/10.1073/pnas.1506295112
- G.E. Belshaw, E. Steer, Y. Ji, H. Azis, B. Sapiie et al., Fluid-rock interaction experiments with andesite at 100 °C for potential carbon storage in geothermal reservoirs. DUSE 3, 369–382 (2024). https://doi.org/10.1002/dug2.12097
- D. He, X. Wang, Y. Yang, R. He, H. Zhong et al., Hydrothermal synthesis of long-chain hydrocarbons up to C24 with NaHCO3-assisted stabilizing cobalt. PNAS 118, e2115059118 (2021). https://doi.org/10.1073/pnas.2115059118
- F. Jin, Y. Gao, Y. Jin, Y. Zhang, J. Cao et al., High-yield reduction of carbon dioxide into formic acid by zero-valent metal/metal oxide redox cycles. Energy Environ. Sci. 4(3), 881 (2011). https://doi.org/10.1039/c0ee00661k
- Z. Ni, H. Zhong, Y. Yang, G. Yao, B. Jin et al., One-step conversion of NaHCO3 into formate and simultaneous synthesis of AlO(OH) from waste Al-can in water. ACS Sustain. Chem. Eng. 7, 5827–5834 (2019). https://doi.org/10.1021/acssuschemeng.8b05681
- J. Song, Y. Yang, G. Yao, H. Zhong, R. He et al., Highly efficient synthesis of hydrogen storage material of formate from bicarbonate and water with general Zn powder. Ind. Eng. Chem. Res. 56(22), 6349–6357 (2017). https://doi.org/10.1021/acs.iecr.7b00190
- X. Zeng, M. Hatakeyama, K. Ogata, J. Liu, Y. Wang et al., New insights into highly efficient reduction of CO2 to formic acid by using zinc under mild hydrothermal conditions: a joint experimental and theoretical study. Phys. Chem. Chem. Phys. 16, 19836–19840 (2014). https://doi.org/10.1039/C4CP03388D
- Z. Tang, W. He, Y. Wang, Y. Wei, X. Yu et al., Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. 311, 121371 (2022). https://doi.org/10.1016/j.apcatb.2022.121371
- J. Yi, R. Xie, Z. Xie, G. Chai, T. Liu et al., Highly selective CO2 electroreduction to CH4 by in situ generated Cu2O single-type sites on a conductive MOF: stabilizing key intermediates with hydrogen bonding. Angew. Chem. Int. Ed. 59, 23641–23648 (2020). https://doi.org/10.1002/anie.202010601
- M. Li, S. Wu, D. Liu, Z. Ye, L. Wang et al., Engineering spatially adjacent redox sites with synergistic spin polarization effect to boost photocatalytic CO2 methanation. J. Am. Chem. Soc. 146(22), 15538–15548 (2024). https://doi.org/10.1021/jacs.4c04264
- S. Wu, X. Tan, J. Lei, H. Chen, L. Wang et al., Ga-doped and Pt-loaded porous TiO2–SiO2 for photocatalytic nonoxidative coupling of methane. J. Am. Chem. Soc. 141, 6592–6600 (2019). https://doi.org/10.1021/jacs.8b13858
- M. Li, S. Wu, D. Liu, Z. Ye, C. He et al., Optimizing reaction kinetics and thermodynamics for photocatalytic CO2 reduction through spin polarization manipulation. ACS Catal. 14(18), 14098–14109 (2024). https://doi.org/10.1021/acscatal.4c03802
- C. He, Q. Li, Z. Ye, L. Wang, Y. Gong et al., Regulating atomically-precise Pt sites for boosting light-driven dry reforming of methane. Angew. Chem. Int. Ed. 63(46), e202412308 (2024). https://doi.org/10.1002/anie.202412308
- J. Wang, D. Liu, M. Li, X. Gu, S. Wu et al., Boosting CO2 photoreduction by synergistic optimization of multiple processes through metal vacancy engineering. Chin. J. Catal. 63, 202–212 (2024). https://doi.org/10.1016/S1872-2067(24)60074-4
- R. Nakamura, T. Takashima, S. Kato, K. Takai, M. Yamamoto et al., Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. 49, 7692–7694 (2010). https://doi.org/10.1002/anie.201003311
- L.M. White, T. Shibuya, S.D. Vance, L.E. Christensen, R. Bhartia et al., Simulating serpentinization as it could apply to the emergence of life using the JPL hydrothermal reactor. Astrobiology 20(3), 307–326 (2020). https://doi.org/10.1089/ast.2018.1949
- G. Proskurowski, M.D. Lilley, J.S. Seewald, G.L. Früh-Green, E.J. Olson et al., Abiogenic hydrocarbon production at lost city hydrothermal field. Science 319, 604–607 (2008). https://doi.org/10.1126/science.1151194
- T.M. McCollom, J.S. Seewald, Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 107(2), 382–401 (2007). https://doi.org/10.1021/cr0503660
- M.J. Russell, Cobalt: A must-have element for life and livelihood. PNAS 119, e2121307119 (2022). https://doi.org/10.1073/pnas.2121307119
- T. Beyazay, C. Ochoa-Hernández, Y. Song, K.S. Belthle, W.F. Martin et al., Influence of composition of nickel-iron nanops for abiotic CO2 conversion to early prebiotic organics. Angew. Chem. Int. Ed. 62(22), e202218189 (2023). https://doi.org/10.1002/anie.202218189
- R. Munirathinam, D. Pham Minh, A. Nzihou, Effect of the support and its surface modifications in cobalt-based Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 57(48), 16137–16161 (2018). https://doi.org/10.1021/acs.iecr.8b03850
- D. Leckel, Diesel production from Fischer–Tropsch: the past, the present, and new concepts. Energy Fuels 23(5), 2342–2358 (2009). https://doi.org/10.1021/ef900064c
- B. Ravel, M. Newville, Athena, Artemis, Hephaestus: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12(4), 537–541 (2005). https://doi.org/10.1107/s0909049505012719
- S.I. Zabinsky, J.J. Rehr, A. Ankudinov, R.C. Albers, M.J. Eller, Multiple-scattering calculations of x-ray-absorption spectra. Phys. Rev. B 52(4), 2995–3009 (1995). https://doi.org/10.1103/physrevb.52.2995
- B. Jongsomjit, C. Sakdamnuson, J.G. Goodwin, P. Praserthdam, Co-support compound formation in titania-supported cobalt catalyst. Catal. Lett. 94(3), 209–215 (2004). https://doi.org/10.1023/B:CATL.0000020548.07021.ec
- A. Kogelbauer, J. Goodwin, R. Oukaci, Ruthenium promotion of Co/Al2O3 Fischer–Tropsch catalysts. J. Catal. 160(1), 125–133 (1996). https://doi.org/10.1006/jcat.1996.0130
- B. Jongsomjit, J. Panpranot, J.G. Goodwin, Co-support compound formation in alumina-supported cobalt catalysts. J. Catal. 204, 98–109 (2001). https://doi.org/10.1006/jcat.2001.3387
- A.K. Yadav, S.M. Haque, D. Shukla, D.M. Phase, S.N. Jha et al., Local structure investigation of Co doped ZnO thin films prepared by RF sputtering technique. Dae solid state physics symposium 2015 Uttar Pradesh, India. Author(s), 060008, 2016. https://doi.org/10.1063/1.4947814
- H. Funke, A.C. Scheinost, M. Chukalina, Wavelet analysis of extended X-ray absorption fine structure data. Phys. Rev. B 71(9), 094110 (2005). https://doi.org/10.1103/physrevb.71.094110
- Y. Dai, M. Xu, Q. Wang, R. Huang, Y. Jin et al., Enhanced activity and stability of Ni/La2O2CO3 catalyst for CO2 methanation by metal-carbonate interaction. Appl. Catal. B Environ. 277, 119271 (2020). https://doi.org/10.1016/j.apcatb.2020.119271
- L. Smoláková, K. Frolich, I. Troppová, P. Kutálek, E. Kroft et al., Determination of basic sites in Mg–Al mixed oxides by combination of TPD-CO2 and CO2 adsorption calorimetry. J. Therm. Anal. Calorim. 127, 1921–1929 (2017). https://doi.org/10.1007/s10973-016-5851-6
- Z. Zhang, S. Xian, H. Xi, H. Wang, Z. Li, Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 66(20), 4878–4888 (2011). https://doi.org/10.1016/j.ces.2011.06.051
- R. Perez, J.M. Brown, Y. Utkin, J. Han, R.F. Curl, Observation of hot bands in the infrared spectrum of H2CO. J. Mol. Spectrosc. 236, 151–157 (2006). https://doi.org/10.1016/j.jms.2006.01.006
References
P. Gai, W. Yu, H. Zhao, R. Qi, F. Li et al., Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid. Angew. Chem. Int. Ed. 59(18), 7224–7229 (2020). https://doi.org/10.1002/anie.202001047
C. Ban, Y. Duan, Y. Wang, J. Ma, K. Wang et al., Isotype heterojunction-boosted CO2 photoreduction to CO. Nano-Micro Lett. 14(1), 74 (2022). https://doi.org/10.1007/s40820-022-00821-9
U. Kang, S.K. Choi, D.J. Ham, S.M. Ji, W. Choi et al., Photosynthesis of formate from CO2 and water at 1% energy efficiency via copper iron oxide catalysis. Energy Environ. Sci. 8(9), 2638–2643 (2015). https://doi.org/10.1039/c5ee01410g
K. Wang, Z. Hu, P. Yu, A.M. Balu, K. Li et al., Understanding bridging sites and accelerating quantum efficiency for photocatalytic CO2 reduction. Nano-Micro Lett. 16(1), 5 (2023). https://doi.org/10.1007/s40820-023-01221-3
X. Meng, T. Wang, L. Liu, S. Ouyang, P. Li et al., Photothermal conversion of CO2 into CH4 with H2 over group VIII nanocatalysts: an alternative approach for solar fuel production. Angew. Chem. Int. Ed. 53, 11478–11482 (2014). https://doi.org/10.1002/anie.201404953
W.C. Chueh, C. Falter, M. Abbott, D. Scipio, P. Furler et al., High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330(6012), 1797–1801 (2010). https://doi.org/10.1126/science.1197834
A. Steinfeld, Solar thermochemical production of hydrogen–– a review. Sol. Energy 78(5), 603–615 (2005). https://doi.org/10.1016/j.solener.2003.12.012
L.O. Schunk, A. Steinfeld, Kinetics of the thermal dissociation of ZnO exposed to concentrated solar irradiation using a solar-driven thermogravimeter in the 1800–2100 K range. AlChE J. 55, 1497–1504 (2009). https://doi.org/10.1002/aic.11765
L.O. Schunk, W. Lipiński, A. Steinfeld, Heat transfer model of a solar receiver-reactor for the thermal dissociation of ZnO: experimental validation at 10 kW and scale-up to 1MW. Chem. Eng. J. 150(2–3), 502–508 (2009). https://doi.org/10.1016/j.cej.2009.03.012
T. Kodama, N. Gokon, Thermochemical cycles for high-temperature solar hydrogen production. Chem. Rev. 107, 4048–4077 (2007). https://doi.org/10.1021/cr050188a
M. Chambon, S. Abanades, G. Flamant, Solar thermal reduction of ZnO and SnO2: characterization of the recombination reaction with O2. Chem. Eng. Sci. 65(11), 3671–3680 (2010). https://doi.org/10.1016/j.ces.2010.03.005
D. Gstoehl, A. Brambilla, L.O. Schunk, A. Steinfeld, A quenching apparatus for the gaseous products of the solar thermal dissociation of ZnO. J. Mater. Sci. 43, 4729–4736 (2008). https://doi.org/10.1007/s10853-007-2351-x
B. Sherwood Lollar, T.D. Westgate, J.A. Ward, G.F. Slater, G. Lacrampe-Couloume, Abiogenic formation of alkanes in the earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 416(6880), 522–524 (2002). https://doi.org/10.1038/416522a
Q. Fu, B. Sherwood Lollar, J. Horita, G. Lacrampe-Couloume, W.E. Seyfried, Abiotic formation of hydrocarbons under hydrothermal conditions: constraints from chemical and isotope data. Geochim. Cosmochim. Acta 71(8), 1982–1998 (2007). https://doi.org/10.1016/j.gca.2007.01.022
J.M. McDermott, J.S. Seewald, C.R. German, S.P. Sylva, Pathways for abiotic organic synthesis at submarine hydrothermal fields. PNAS 112, 7668–7672 (2015). https://doi.org/10.1073/pnas.1506295112
G.E. Belshaw, E. Steer, Y. Ji, H. Azis, B. Sapiie et al., Fluid-rock interaction experiments with andesite at 100 °C for potential carbon storage in geothermal reservoirs. DUSE 3, 369–382 (2024). https://doi.org/10.1002/dug2.12097
D. He, X. Wang, Y. Yang, R. He, H. Zhong et al., Hydrothermal synthesis of long-chain hydrocarbons up to C24 with NaHCO3-assisted stabilizing cobalt. PNAS 118, e2115059118 (2021). https://doi.org/10.1073/pnas.2115059118
F. Jin, Y. Gao, Y. Jin, Y. Zhang, J. Cao et al., High-yield reduction of carbon dioxide into formic acid by zero-valent metal/metal oxide redox cycles. Energy Environ. Sci. 4(3), 881 (2011). https://doi.org/10.1039/c0ee00661k
Z. Ni, H. Zhong, Y. Yang, G. Yao, B. Jin et al., One-step conversion of NaHCO3 into formate and simultaneous synthesis of AlO(OH) from waste Al-can in water. ACS Sustain. Chem. Eng. 7, 5827–5834 (2019). https://doi.org/10.1021/acssuschemeng.8b05681
J. Song, Y. Yang, G. Yao, H. Zhong, R. He et al., Highly efficient synthesis of hydrogen storage material of formate from bicarbonate and water with general Zn powder. Ind. Eng. Chem. Res. 56(22), 6349–6357 (2017). https://doi.org/10.1021/acs.iecr.7b00190
X. Zeng, M. Hatakeyama, K. Ogata, J. Liu, Y. Wang et al., New insights into highly efficient reduction of CO2 to formic acid by using zinc under mild hydrothermal conditions: a joint experimental and theoretical study. Phys. Chem. Chem. Phys. 16, 19836–19840 (2014). https://doi.org/10.1039/C4CP03388D
Z. Tang, W. He, Y. Wang, Y. Wei, X. Yu et al., Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. 311, 121371 (2022). https://doi.org/10.1016/j.apcatb.2022.121371
J. Yi, R. Xie, Z. Xie, G. Chai, T. Liu et al., Highly selective CO2 electroreduction to CH4 by in situ generated Cu2O single-type sites on a conductive MOF: stabilizing key intermediates with hydrogen bonding. Angew. Chem. Int. Ed. 59, 23641–23648 (2020). https://doi.org/10.1002/anie.202010601
M. Li, S. Wu, D. Liu, Z. Ye, L. Wang et al., Engineering spatially adjacent redox sites with synergistic spin polarization effect to boost photocatalytic CO2 methanation. J. Am. Chem. Soc. 146(22), 15538–15548 (2024). https://doi.org/10.1021/jacs.4c04264
S. Wu, X. Tan, J. Lei, H. Chen, L. Wang et al., Ga-doped and Pt-loaded porous TiO2–SiO2 for photocatalytic nonoxidative coupling of methane. J. Am. Chem. Soc. 141, 6592–6600 (2019). https://doi.org/10.1021/jacs.8b13858
M. Li, S. Wu, D. Liu, Z. Ye, C. He et al., Optimizing reaction kinetics and thermodynamics for photocatalytic CO2 reduction through spin polarization manipulation. ACS Catal. 14(18), 14098–14109 (2024). https://doi.org/10.1021/acscatal.4c03802
C. He, Q. Li, Z. Ye, L. Wang, Y. Gong et al., Regulating atomically-precise Pt sites for boosting light-driven dry reforming of methane. Angew. Chem. Int. Ed. 63(46), e202412308 (2024). https://doi.org/10.1002/anie.202412308
J. Wang, D. Liu, M. Li, X. Gu, S. Wu et al., Boosting CO2 photoreduction by synergistic optimization of multiple processes through metal vacancy engineering. Chin. J. Catal. 63, 202–212 (2024). https://doi.org/10.1016/S1872-2067(24)60074-4
R. Nakamura, T. Takashima, S. Kato, K. Takai, M. Yamamoto et al., Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. 49, 7692–7694 (2010). https://doi.org/10.1002/anie.201003311
L.M. White, T. Shibuya, S.D. Vance, L.E. Christensen, R. Bhartia et al., Simulating serpentinization as it could apply to the emergence of life using the JPL hydrothermal reactor. Astrobiology 20(3), 307–326 (2020). https://doi.org/10.1089/ast.2018.1949
G. Proskurowski, M.D. Lilley, J.S. Seewald, G.L. Früh-Green, E.J. Olson et al., Abiogenic hydrocarbon production at lost city hydrothermal field. Science 319, 604–607 (2008). https://doi.org/10.1126/science.1151194
T.M. McCollom, J.S. Seewald, Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 107(2), 382–401 (2007). https://doi.org/10.1021/cr0503660
M.J. Russell, Cobalt: A must-have element for life and livelihood. PNAS 119, e2121307119 (2022). https://doi.org/10.1073/pnas.2121307119
T. Beyazay, C. Ochoa-Hernández, Y. Song, K.S. Belthle, W.F. Martin et al., Influence of composition of nickel-iron nanops for abiotic CO2 conversion to early prebiotic organics. Angew. Chem. Int. Ed. 62(22), e202218189 (2023). https://doi.org/10.1002/anie.202218189
R. Munirathinam, D. Pham Minh, A. Nzihou, Effect of the support and its surface modifications in cobalt-based Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 57(48), 16137–16161 (2018). https://doi.org/10.1021/acs.iecr.8b03850
D. Leckel, Diesel production from Fischer–Tropsch: the past, the present, and new concepts. Energy Fuels 23(5), 2342–2358 (2009). https://doi.org/10.1021/ef900064c
B. Ravel, M. Newville, Athena, Artemis, Hephaestus: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12(4), 537–541 (2005). https://doi.org/10.1107/s0909049505012719
S.I. Zabinsky, J.J. Rehr, A. Ankudinov, R.C. Albers, M.J. Eller, Multiple-scattering calculations of x-ray-absorption spectra. Phys. Rev. B 52(4), 2995–3009 (1995). https://doi.org/10.1103/physrevb.52.2995
B. Jongsomjit, C. Sakdamnuson, J.G. Goodwin, P. Praserthdam, Co-support compound formation in titania-supported cobalt catalyst. Catal. Lett. 94(3), 209–215 (2004). https://doi.org/10.1023/B:CATL.0000020548.07021.ec
A. Kogelbauer, J. Goodwin, R. Oukaci, Ruthenium promotion of Co/Al2O3 Fischer–Tropsch catalysts. J. Catal. 160(1), 125–133 (1996). https://doi.org/10.1006/jcat.1996.0130
B. Jongsomjit, J. Panpranot, J.G. Goodwin, Co-support compound formation in alumina-supported cobalt catalysts. J. Catal. 204, 98–109 (2001). https://doi.org/10.1006/jcat.2001.3387
A.K. Yadav, S.M. Haque, D. Shukla, D.M. Phase, S.N. Jha et al., Local structure investigation of Co doped ZnO thin films prepared by RF sputtering technique. Dae solid state physics symposium 2015 Uttar Pradesh, India. Author(s), 060008, 2016. https://doi.org/10.1063/1.4947814
H. Funke, A.C. Scheinost, M. Chukalina, Wavelet analysis of extended X-ray absorption fine structure data. Phys. Rev. B 71(9), 094110 (2005). https://doi.org/10.1103/physrevb.71.094110
Y. Dai, M. Xu, Q. Wang, R. Huang, Y. Jin et al., Enhanced activity and stability of Ni/La2O2CO3 catalyst for CO2 methanation by metal-carbonate interaction. Appl. Catal. B Environ. 277, 119271 (2020). https://doi.org/10.1016/j.apcatb.2020.119271
L. Smoláková, K. Frolich, I. Troppová, P. Kutálek, E. Kroft et al., Determination of basic sites in Mg–Al mixed oxides by combination of TPD-CO2 and CO2 adsorption calorimetry. J. Therm. Anal. Calorim. 127, 1921–1929 (2017). https://doi.org/10.1007/s10973-016-5851-6
Z. Zhang, S. Xian, H. Xi, H. Wang, Z. Li, Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 66(20), 4878–4888 (2011). https://doi.org/10.1016/j.ces.2011.06.051
R. Perez, J.M. Brown, Y. Utkin, J. Han, R.F. Curl, Observation of hot bands in the infrared spectrum of H2CO. J. Mol. Spectrosc. 236, 151–157 (2006). https://doi.org/10.1016/j.jms.2006.01.006