One Step Quick Detection of Cancer Cell Surface Marker by Integrated NiFe-based Magnetic Biosensing Cell Cultural Chip
Corresponding Author: Yong Zhou
Nano-Micro Letters,
Vol. 5 No. 3 (2013), Article Number: 213-222
Abstract
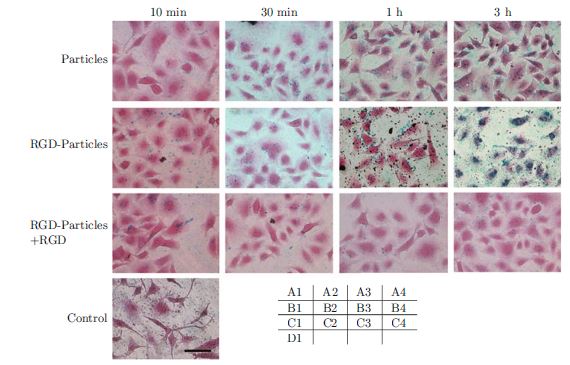
RGD peptides has been used to detect cell surface integrin and direct clinical effective therapeutic drug selection. Herein we report that a quick one step detection of cell surface marker that was realized by a specially designed NiFe-based magnetic biosensing cell chip combined with functionalized magnetic nanoparticles. Magnetic nanoparticles with 20-30 nm in diameter were prepared by coprecipitation and modified with RGD-4C, and the resultant RGD-functionalized magnetic nanoparticles were used for targeting cancer cells cultured on the NiFe-based magnetic biosensing chip and distinguish the amount of cell surface receptor-integrin. Cell lines such as Calu3, Hela, A549, CaFbr, HEK293 and HUVEC exhibiting different integrin expression were chosen as test samples. Calu3, Hela, HEK293 and HUVEC cells were successfully identified. This approach has advantages in the qualitative screening test. Compared with traditional method, it is fast, sensitive, low cost, easy-operative, and needs very little human intervention. The novel method has great potential in applications such as fast clinical cell surface marker detection, and diagnosis of early cancer, and can be easily extended to other biomedical applications based on molecular recognition.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- C. Gui, X. Dai and D. Cui, “Advances of nanotech- nology applied to biosensors”, Nano Biomed. Eng. 3(4), 260–273 (2011). http://dx.doi.org/10.5101/nbe.v3i4.p260-273
- J. Chen, D. Chen, T. Yuan and X. Chen, “Microfluidic PCR Chips”, Nano Biomed. Eng. 3 (4), 203–210 (2011). http://dx.doi.org/10.5101/nbe.v3i4.p203-210
- R. Vijayakumar, S. Jagannathan, P. R. Gandhi and S. Chaansha, “Nanorobotics: a newer platform for molecular diagnose”, Nano Biomed. Eng. 3(3), 192–201 (2011). http://dx.doi.org/10.5101/nbe.v3i3.p192-201
- X. Zhang, L. Jiang, C. Zhang, D. Li, C. Wang, F. Gao and D. Cui, “A silicon dioxide modified magnetic nanoparticles labeled lateral flow strips for HBs antigen”, J. Biomed. Nanotechnol. 7(6), 776–781 (2011). http://dx.doi.org/10.1166/jbn.2011.1352
- R. Bashir, “BioMEMS: state-of-the-art in detection, opportunities and prospects”, Adv. Drug Deliv. Rev. 56(11), 1565–1586 (2004). http://dx.doi.org/10.1016/j.addr.2004.03.002.
- D. R. Baselt, G. U. Lee, M. Natesan, S.W. Metzger, P. E. Sheehan and R. J. Colton, “A biosensor based on magnetoresistance technology”, Biosens. Bioelectron. 13(7), 731–739 (1998). http://dx.doi.org/10.1016/S0956-5663(98)00037-2
- G. Kurlyandskaya and V. Levit, “Magnetic Dynabeads detection by sensitive element based on giant magnetoimpedance”, Biosens. Bioelectron. 20(8), 1611–1616 (2005). http://dx.doi.org/10.1016/j.bios.2004.04.027
- S. Baranov, “Magnetic models of cast amorphous microwires”, Surf. Eng. Appl. Electrochem. 47(4), 308–322 (2011). http://dx.doi.org/10.3103/S1068375511040028
- F. Blanc-Beguin, S. Nabily, J. Gieraltowski, A. Turzo, S. Querellou and P. Salaun, “Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells”, J. Magn. Magn. Mater. 321(3), 192–197 (2009). http://dx.doi.org/10.1016/j.jmmm.2008.08.104
- H. Yang, L. Chen, C. Lei, J. Zhang, D. Li, Z. M. Zhou, C. C. Bao, H. Y. Hu, X. Chen and F. Cui, “Giant magnetoimpedance-based microchannel system for quick and parallel genotyping of human papilloma virus type 16/18”, Appl. Phys. Lett. 97 (4), 043702-043702-3 (2010). http://dx.doi.org/10.1063/1.3467833
- X. Zhi, Q. Liu, X. Zhang, Y. Zhang, J. Feng and D. Cui, “Quick genotyping detection of HBV by giant magnetoresistive biochip combined with PCR and line probe assay”, Lab Chip. 12(4), 741 (2012). http://dx.doi.org/10.1039/c2lc20949g
- L. Chen, C.-C. Bao, H. Yang, D. Li, C. Lei, T. Wang, H.-Y. Hu, M. He, Y. Zhou and D.-X. Cui, “A prototype of giant magnetoimpedance-based biosensing system for targeted detection of gastric cancer cells”, Biosens. Bioelectron. 26(7), 3246–3253 (2011). http://dx.doi.org/10.1016/j.bios.2010.12.034
- G. Kurlyandskaya, “Giant magnetoimpedance for biosensing: Advantages and shortcomings”, J. Magn. Magn. Mater. 321(7), 659–662 (2009). http://dx.doi.org/10.1016/j.jmmm.2008.11.019
- L. Chen, Y. Zhou, C. Lei, Z. Zhou and W. Ding, “Giant magnetoimpedance effect in sputtered single layered NiFe film and meander NiFe/Cu/NiFe film”, J. Magn. Magn. Mater. 322(19), 2834–2839 (2010). http://dx.doi.org/10.1016/j.jmmm.2010.04.038
- S. Petersen, J. M. Alonso, A. Specht, P. Duodu, M. Goeldner and A. delCampo, “Phototriggering of Cell Adhesion by Caged Cyclic RGD Peptides”, Angew. Chem. Int. Ed. 47(17), 3192–3195 (2008). http://dx.doi.org/10.1002/anie.200704857
- X. Chen, “Multimodality imaging of tumor integrin alphavbeta3 expression”, Mini-Rev. Med. Chem. 6(2), 227 (2006). http://dx.doi.org/10.2174/138955706775475975
- H. D. Han, L. S. Mangala, J. W. Lee, M. M. K. Shahzad, H. S. Kim, D. Shen, E. J. Nam, E. M. Mora, R. L. Stone, C. Lu, S. J. Lee, J.W. Roh, A.M. Nick, G. Lopez-Berestein and A. K. Sood, “Targeted Gene Silencing Using RGD-Labeled Chitosan Nanoparticles”, Clin. Cancer Res. 16(15), 3910–3922 (2010). http://dx.doi.org/10.1158/1078-0432.ccr-10-0005.
- X. Montet, K. Montet-Abou, F. Reynolds, R. Weissleder and L. Josephson, “Nanoparticle imaging of integrins on tumor cells”, Neoplasia (New York, NY). 8(3), 214 (2006). http://dx.doi.org/10.1593/neo.05769
- Z. Li, P. Huang, X. Zhang, J. Lin, S. Yang, B. Liu, F. Gao, P. Xi, Q. Ren and D. Cui, “RGD- conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy”, Mol. Pharm. 7(1), 94–104 (2009). http://dx.doi.org/10.1021/mp9001415
- Y. Ye, S. Bloch, B. Xu and S. Achilefu, “Design, synthesis, and evaluation of near infrared fluorescent multimeric RGD peptides for targeting tumors”, J. Med. Chem. 49(7), 2268–2275 (2006). http://dx.doi.org/10.1021/jm050947h
- I. Schmid, C. H. Uittenbogaart, B. Keld and J. V. Giorgi, “A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry”, J. Immunol. Methods. 170(2), 145 (1994). http://dx.doi.org/10.1016/0022-1759(94)90390-5
- C. Bao, N. Beziere, P. del Pino, B. Pelaz, G. Estrada, F. Tian, V. Ntziachristos, J. M. de la Fuente and D. Cui, “Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers”, Small 9(1), 68–74 (2013). http://dx.doi.org/10.1002/smll.201201779
- H. Hu, H. Yang, D. Li, K. Wang, J. Ruan, X. Zhang, J. Chen, C. Bao, J. Ji and D. Shi, “The potential of magnetic nanocluster and dual-functional protein-based strategy for noninvasive detection of HBV surface antibodies”, Analyst. 136(4), 679–683 (2010). http://dx.doi.org/10.1039/c0an00517g
- B. Pan, D. Cui, Y. Sheng, C. Ozkan, F. Gao, R. He, Q. Li, P. Xu and T. Huang, “Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system”, Cancer research. 67(17), 8156–8163 (2007). http://dx.doi.org/10.1158/0008-5472.CAN-06-4762
- G. Gao, P. Huang, Y. Zhang, K. Wang, W. Qin and D. Cui, “Gram scale synthesis of superparamagnetic Fe3O4 nanoparticles and fluid via a facile solvothermal route”, Crystengcomm. 13(6), 1782–1785 (2011). http://dx.doi.org/10.1039/c0ce00584c
- G. Gao, P. Huang, K. Wang, R. He and D. Cui, “Gramscale synthesis and shape evolution of micro-CaCO3”, Powder Technol. 205(1), 270–275 (2011). http://dx.doi.org/10.1016/j.powtec.2010.09.032
- H. Hu, H. Yang, P. Huang, D. Cui, Y. Peng, J. Zhang, F. Lu, J. Lian and D. Shi, “Unique role of ionic liquid in microwave-assisted synthesis of monodisperse magnetite nanoparticles”, Chem. Commun. 46(22), 3866–3868 (2010). http://dx.doi.org/10.1039/b927321b
- C. Zhang, M. Jugold, E. C. Woenne, T. Lammers, B. Morgenstern, M. M. Mueller, H. Zentgraf, M. Bock, M. Eisenhut and W. Semmler, “Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner”, Cancer Res. 67(4), 1555–1562 (2007). http://dx.doi.org/10.1158/0008-5472.CAN-06-1668
- G. Thumshirn, U. Hersel, S. L. Goodman and H. Kessler, “Multimeric cyclic RGD peptides as poten- tial tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation”, Chem. Eur. J. 9(12), 2717–2725 (2003). http://dx.doi.org/10.1002/chem.200204304
- E. D. Hay, “Collagen and other matrix glycoproteins in embryogenesis”, Cell biology of extracellular matrix 2, 419–462 (1991).
- J. Madri, “Endothelial cell-matrix interactions in hemostasis”, Progress in hemostasis and thrombosis. 6, 1 (1982).
- U. Saarialho-Kere, S. Kovacs, A. Pentland, J. Olerud, H. Welgus and W. Parks, “Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing”, J. Clin. Invest. 92(6), 2858–2866 (1993). http://dx.doi.org/10.1172/JCI116906
- G. G. Vaday and O. Lider, “Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation”, J. Leukoc. Biol. 67(2), 149–159 (2000).
References
C. Gui, X. Dai and D. Cui, “Advances of nanotech- nology applied to biosensors”, Nano Biomed. Eng. 3(4), 260–273 (2011). http://dx.doi.org/10.5101/nbe.v3i4.p260-273
J. Chen, D. Chen, T. Yuan and X. Chen, “Microfluidic PCR Chips”, Nano Biomed. Eng. 3 (4), 203–210 (2011). http://dx.doi.org/10.5101/nbe.v3i4.p203-210
R. Vijayakumar, S. Jagannathan, P. R. Gandhi and S. Chaansha, “Nanorobotics: a newer platform for molecular diagnose”, Nano Biomed. Eng. 3(3), 192–201 (2011). http://dx.doi.org/10.5101/nbe.v3i3.p192-201
X. Zhang, L. Jiang, C. Zhang, D. Li, C. Wang, F. Gao and D. Cui, “A silicon dioxide modified magnetic nanoparticles labeled lateral flow strips for HBs antigen”, J. Biomed. Nanotechnol. 7(6), 776–781 (2011). http://dx.doi.org/10.1166/jbn.2011.1352
R. Bashir, “BioMEMS: state-of-the-art in detection, opportunities and prospects”, Adv. Drug Deliv. Rev. 56(11), 1565–1586 (2004). http://dx.doi.org/10.1016/j.addr.2004.03.002.
D. R. Baselt, G. U. Lee, M. Natesan, S.W. Metzger, P. E. Sheehan and R. J. Colton, “A biosensor based on magnetoresistance technology”, Biosens. Bioelectron. 13(7), 731–739 (1998). http://dx.doi.org/10.1016/S0956-5663(98)00037-2
G. Kurlyandskaya and V. Levit, “Magnetic Dynabeads detection by sensitive element based on giant magnetoimpedance”, Biosens. Bioelectron. 20(8), 1611–1616 (2005). http://dx.doi.org/10.1016/j.bios.2004.04.027
S. Baranov, “Magnetic models of cast amorphous microwires”, Surf. Eng. Appl. Electrochem. 47(4), 308–322 (2011). http://dx.doi.org/10.3103/S1068375511040028
F. Blanc-Beguin, S. Nabily, J. Gieraltowski, A. Turzo, S. Querellou and P. Salaun, “Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells”, J. Magn. Magn. Mater. 321(3), 192–197 (2009). http://dx.doi.org/10.1016/j.jmmm.2008.08.104
H. Yang, L. Chen, C. Lei, J. Zhang, D. Li, Z. M. Zhou, C. C. Bao, H. Y. Hu, X. Chen and F. Cui, “Giant magnetoimpedance-based microchannel system for quick and parallel genotyping of human papilloma virus type 16/18”, Appl. Phys. Lett. 97 (4), 043702-043702-3 (2010). http://dx.doi.org/10.1063/1.3467833
X. Zhi, Q. Liu, X. Zhang, Y. Zhang, J. Feng and D. Cui, “Quick genotyping detection of HBV by giant magnetoresistive biochip combined with PCR and line probe assay”, Lab Chip. 12(4), 741 (2012). http://dx.doi.org/10.1039/c2lc20949g
L. Chen, C.-C. Bao, H. Yang, D. Li, C. Lei, T. Wang, H.-Y. Hu, M. He, Y. Zhou and D.-X. Cui, “A prototype of giant magnetoimpedance-based biosensing system for targeted detection of gastric cancer cells”, Biosens. Bioelectron. 26(7), 3246–3253 (2011). http://dx.doi.org/10.1016/j.bios.2010.12.034
G. Kurlyandskaya, “Giant magnetoimpedance for biosensing: Advantages and shortcomings”, J. Magn. Magn. Mater. 321(7), 659–662 (2009). http://dx.doi.org/10.1016/j.jmmm.2008.11.019
L. Chen, Y. Zhou, C. Lei, Z. Zhou and W. Ding, “Giant magnetoimpedance effect in sputtered single layered NiFe film and meander NiFe/Cu/NiFe film”, J. Magn. Magn. Mater. 322(19), 2834–2839 (2010). http://dx.doi.org/10.1016/j.jmmm.2010.04.038
S. Petersen, J. M. Alonso, A. Specht, P. Duodu, M. Goeldner and A. delCampo, “Phototriggering of Cell Adhesion by Caged Cyclic RGD Peptides”, Angew. Chem. Int. Ed. 47(17), 3192–3195 (2008). http://dx.doi.org/10.1002/anie.200704857
X. Chen, “Multimodality imaging of tumor integrin alphavbeta3 expression”, Mini-Rev. Med. Chem. 6(2), 227 (2006). http://dx.doi.org/10.2174/138955706775475975
H. D. Han, L. S. Mangala, J. W. Lee, M. M. K. Shahzad, H. S. Kim, D. Shen, E. J. Nam, E. M. Mora, R. L. Stone, C. Lu, S. J. Lee, J.W. Roh, A.M. Nick, G. Lopez-Berestein and A. K. Sood, “Targeted Gene Silencing Using RGD-Labeled Chitosan Nanoparticles”, Clin. Cancer Res. 16(15), 3910–3922 (2010). http://dx.doi.org/10.1158/1078-0432.ccr-10-0005.
X. Montet, K. Montet-Abou, F. Reynolds, R. Weissleder and L. Josephson, “Nanoparticle imaging of integrins on tumor cells”, Neoplasia (New York, NY). 8(3), 214 (2006). http://dx.doi.org/10.1593/neo.05769
Z. Li, P. Huang, X. Zhang, J. Lin, S. Yang, B. Liu, F. Gao, P. Xi, Q. Ren and D. Cui, “RGD- conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy”, Mol. Pharm. 7(1), 94–104 (2009). http://dx.doi.org/10.1021/mp9001415
Y. Ye, S. Bloch, B. Xu and S. Achilefu, “Design, synthesis, and evaluation of near infrared fluorescent multimeric RGD peptides for targeting tumors”, J. Med. Chem. 49(7), 2268–2275 (2006). http://dx.doi.org/10.1021/jm050947h
I. Schmid, C. H. Uittenbogaart, B. Keld and J. V. Giorgi, “A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry”, J. Immunol. Methods. 170(2), 145 (1994). http://dx.doi.org/10.1016/0022-1759(94)90390-5
C. Bao, N. Beziere, P. del Pino, B. Pelaz, G. Estrada, F. Tian, V. Ntziachristos, J. M. de la Fuente and D. Cui, “Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers”, Small 9(1), 68–74 (2013). http://dx.doi.org/10.1002/smll.201201779
H. Hu, H. Yang, D. Li, K. Wang, J. Ruan, X. Zhang, J. Chen, C. Bao, J. Ji and D. Shi, “The potential of magnetic nanocluster and dual-functional protein-based strategy for noninvasive detection of HBV surface antibodies”, Analyst. 136(4), 679–683 (2010). http://dx.doi.org/10.1039/c0an00517g
B. Pan, D. Cui, Y. Sheng, C. Ozkan, F. Gao, R. He, Q. Li, P. Xu and T. Huang, “Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system”, Cancer research. 67(17), 8156–8163 (2007). http://dx.doi.org/10.1158/0008-5472.CAN-06-4762
G. Gao, P. Huang, Y. Zhang, K. Wang, W. Qin and D. Cui, “Gram scale synthesis of superparamagnetic Fe3O4 nanoparticles and fluid via a facile solvothermal route”, Crystengcomm. 13(6), 1782–1785 (2011). http://dx.doi.org/10.1039/c0ce00584c
G. Gao, P. Huang, K. Wang, R. He and D. Cui, “Gramscale synthesis and shape evolution of micro-CaCO3”, Powder Technol. 205(1), 270–275 (2011). http://dx.doi.org/10.1016/j.powtec.2010.09.032
H. Hu, H. Yang, P. Huang, D. Cui, Y. Peng, J. Zhang, F. Lu, J. Lian and D. Shi, “Unique role of ionic liquid in microwave-assisted synthesis of monodisperse magnetite nanoparticles”, Chem. Commun. 46(22), 3866–3868 (2010). http://dx.doi.org/10.1039/b927321b
C. Zhang, M. Jugold, E. C. Woenne, T. Lammers, B. Morgenstern, M. M. Mueller, H. Zentgraf, M. Bock, M. Eisenhut and W. Semmler, “Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner”, Cancer Res. 67(4), 1555–1562 (2007). http://dx.doi.org/10.1158/0008-5472.CAN-06-1668
G. Thumshirn, U. Hersel, S. L. Goodman and H. Kessler, “Multimeric cyclic RGD peptides as poten- tial tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation”, Chem. Eur. J. 9(12), 2717–2725 (2003). http://dx.doi.org/10.1002/chem.200204304
E. D. Hay, “Collagen and other matrix glycoproteins in embryogenesis”, Cell biology of extracellular matrix 2, 419–462 (1991).
J. Madri, “Endothelial cell-matrix interactions in hemostasis”, Progress in hemostasis and thrombosis. 6, 1 (1982).
U. Saarialho-Kere, S. Kovacs, A. Pentland, J. Olerud, H. Welgus and W. Parks, “Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing”, J. Clin. Invest. 92(6), 2858–2866 (1993). http://dx.doi.org/10.1172/JCI116906
G. G. Vaday and O. Lider, “Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation”, J. Leukoc. Biol. 67(2), 149–159 (2000).