Selection of Negative Charged Acidic Polar Additives to Regulate Electric Double Layer for Stable Zinc Ion Battery
Corresponding Author: Jiang Zhou
Nano-Micro Letters,
Vol. 16 (2024), Article Number: 270
Abstract
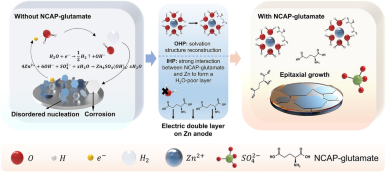
Zinc-ion batteries are promising for large-scale electrochemical energy storage systems, which still suffer from interfacial issues, e.g., hydrogen evolution side reaction (HER), self-corrosion, and uncontrollable dendritic Zn electrodeposition. Although the regulation of electric double layer (EDL) has been verified for interfacial issues, the principle to select the additive as the regulator is still misted. Here, several typical amino acids with different characteristics were examined to reveal the interfacial behaviors in regulated EDL on the Zn anode. Negative charged acidic polarity (NCAP) has been unveiled as the guideline for selecting additive to reconstruct EDL with an inner zincophilic H2O-poor layer and to replace H2O molecules of hydrated Zn2+ with NCAP glutamate. Taking the synergistic effects of EDL regulation, the uncontrollable interface is significantly stabilized from the suppressed HER and anti-self-corrosion with uniform electrodeposition. Consequently, by adding NCAP glutamate, a high average Coulombic efficiency of 99.83% of Zn metal is achieved in Zn|Cu asymmetrical cell for over 2000 cycles, and NH4V4O10|Zn full cell exhibits a high-capacity retention of 82.1% after 3000 cycles at 2 A g−1. Recapitulating, the NCAP principle posted here can quicken the design of trailblazing electrolyte additives for aqueous Zn-based electrochemical energy storage systems.
Highlights:
1 Negative charged acidic polarity (NCAP) has been unveiled as the guideline for selecting additives to regulate electric double layer (EDL) for Zn-ion batteries.
2 NCAP glutamate has been verified to regulate EDL structure with synergetic effects, including preferential adsorption on Zn anode and reconstruction of hydrated Zn-ion clusters.
3 Adding NCAP additives, Zn|Cu half-cell achieves a high Coulombic efficiency of 99.83% for 2000 cycles, and NH4V4O10|Zn full cell realizes a high-capacity retention of 82.1% for 3000 cycles.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- F. Wang, J. Xie, D. Zheng, F. Yang, H. Zhang et al., Intrinsic carbon defects induced reversible antimony chemistry for high-energy aqueous alkaline batteries. Adv. Mater. 34, 2200085 (2022). https://doi.org/10.1002/adma.202200085
- D. Li, Y. Guo, C. Zhang, X. Chen, W. Zhang et al., Unveiling organic electrode materials in aqueous zinc-ion batteries: from structural design to electrochemical performance. Nano-Micro Lett. 16, 194 (2024). https://doi.org/10.1007/s40820-024-01404-6
- W. Li, Z. Ji, F. Dong, Y. Yang, Evaluation of provincial renewable energy generation efficiency and spatio-temporal heterogeneity of influencing factors in China. Renew. Energy 226, 120446 (2024). https://doi.org/10.1016/j.renene.2024.120446
- Y. Huang, Q. Gu, Z. Guo, W. Liu, Z. Chang et al., Unraveling dynamical behaviors of zinc metal electrodes in aqueous electrolytes through an operando study. Energy Storage Mater. 46, 243–251 (2022). https://doi.org/10.1016/j.ensm.2022.01.012
- M. Han, D. Chen, Q. Lu, G. Fang, Aqueous rechargeable Zn–iodine batteries: issues, strategies and perspectives. Small 20, 2310293 (2023). https://doi.org/10.1002/smll.202310293
- A. Zhou, H. Wang, F. Zhang, X. Hu, Z. Song et al., Amphipathic phenylalanine-induced nucleophilic-hydrophobic interface toward highly reversible Zn anode. Nano-Micro Lett. 16, 164 (2024). https://doi.org/10.1007/s40820-024-01380-x
- X. Xie, J. Li, Z. Xing, B. Lu, S. Liang et al., Biocompatible zinc battery with programmable electro-cross-linked electrolyte. Natl. Sci. Rev. 10, nwac281 (2023). https://doi.org/10.1093/nsr/nwac281
- X. Chen, X. Xie, P. Ruan, S. Liang, W.-Y. Wong et al., Thermodynamics and kinetics of conversion reaction in zinc batteries. ACS Energy Lett. 9, 2037–2056 (2024). https://doi.org/10.1021/acsenergylett.4c00450
- M. Zhang, W. Xu, X. Han, H. Fan, T. Chen et al., Unveiling the mechanism of the dendrite nucleation and growth in aqueous zinc ion batteries. Adv. Energy Mater. 14, 2303737 (2023). https://doi.org/10.1002/aenm.202303737
- Q. Zou, Z. Liang, W. Wang, D. Dong, Y.-C. Lu, A nuclei-rich strategy for highly reversible dendrite-free zinc metal anodes. Energy Environ. Sci. 16, 6026–6034 (2023). https://doi.org/10.1039/d3ee03246a
- Z. Xiang, Y. Qiu, X. Guo, K. Qi, Z.-L. Xu et al., Inherited construction of porous zinc hydroxide sulfate layer for stable dendrite-free Zn anode. Energy Environ. Sci. 17, 3409–3418 (2024). https://doi.org/10.1039/d4ee00721b
- Z. Khan, D. Kumar, X. Crispin, Does water-in-salt electrolyte subdue issues of Zn batteries? Adv. Mater. 35, 2300369 (2023). https://doi.org/10.1002/adma.202300369
- Y. Liu, H. He, A. Gao, J. Ling, F. Yi et al., Fundamental study on Zn corrosion and dendrite growth in gel electrolyte towards advanced wearable Zn-ion battery. Chem. Eng. J. 446, 137021 (2022). https://doi.org/10.1016/j.cej.2022.137021
- C. Huang, X. Zhao, Y. Hao, Y. Yang, Y. Qian et al., Selection criteria for electrical double layer structure regulators enabling stable Zn metal anodes. Energy Environ. Sci. 16, 1721–1731 (2023). https://doi.org/10.1039/d3ee00045a
- T.C. Li, C. Lin, M. Luo, P. Wang, D.-S. Li et al., Interfacial molecule engineering for reversible Zn electrochemistry. ACS Energy Lett. 8, 3258–3268 (2023). https://doi.org/10.1021/acsenergylett.3c00859
- J. Wu, Understanding the electric double-layer structure, capacitance, and charging dynamics. Chem. Rev. 122, 10821–10859 (2022). https://doi.org/10.1021/acs.chemrev.2c00097
- W. Nie, H. Cheng, Q. Sun, S. Liang, X. Lu et al., Design strategies toward high-performance Zn metal anode. Small Methods 8, e2201572 (2024). https://doi.org/10.1002/smtd.202201572
- T. Wang, Y. Tang, M. Yu, B. Lu, X. Zhang et al., Spirally grown zinc-cobalt alloy layer enables highly reversible zinc metal anodes. Adv. Funct. Mater. 33, 2306101 (2023). https://doi.org/10.1002/adfm.202306101
- Y. Zhu, G. Liang, X. Cui, X. Liu, H. Zhong et al., Engineering hosts for Zn anodes in aqueous Zn-ion batteries. Energy Environ. Sci. 17, 369–385 (2024). https://doi.org/10.1039/d3ee03584k
- J. Chen, H. Zhang, M. Fang, C. Ke, S. Liu et al., Design of localized high-concentration electrolytes via donor number. ACS Energy Lett. 8, 1723–1734 (2023). https://doi.org/10.1021/acsenergylett.3c00004
- Y. Xu, X. Zhou, Z. Chen, Y. Hou, Y. You et al., Electrolyte formulas of aqueous zinc ion battery: a physical difference with chemical consequences. Mater. Today 66, 339–347 (2023). https://doi.org/10.1016/j.mattod.2023.04.005
- M. Han, T.C. Li, X. Chen, H.Y. Yang, Electrolyte modulation strategies for low-temperature Zn batteries. Small 20, 2304901 (2023). https://doi.org/10.1002/smll.202304901
- J. Weng, W. Zhu, K. Yu, J. Luo, M. Chen et al., Enhancing Zn-metal anode stability: key effects of electrolyte additives on ion-shield-like electrical double layer and stable solid electrolyte interphase. Adv. Funct. Mater. 34, 2314347 (2024). https://doi.org/10.1002/adfm.202314347
- Y. Liu, B. Xie, Q. Hu, R. Zhao, Q. Zheng et al., Regulating the helmholtz plane by trace polarity additive for long-life Zn ion batteries. Energy Storage Mater. 66, 103202 (2024). https://doi.org/10.1016/j.ensm.2024.103202
- X. Li, M. Wang, Y. Chu, Y. Gao, Z. Yang et al., Modulation of water reactivity by ethyl acetate/water co-solvent for zinc-metal batteries. Chem. Eng. J. 487, 150588 (2024). https://doi.org/10.1016/j.cej.2024.150588
- Y. Yang, Y. Li, Q. Zhu, B. Xu, Optimal molecular configuration of electrolyte additives enabling stabilization of zinc anodes. Adv. Funct. Mater. (2024). https://doi.org/10.1002/adfm.202316371
- Q. Meng, Q. Bai, R. Zhao, P. Cao, G. Zhang et al., Attenuating water activity through impeded proton transfer resulting from hydrogen bond enhancement effect for fast and ultra-stable Zn metal anode. Adv. Energy Mater. 13, 2302828 (2023). https://doi.org/10.1002/aenm.202302828
- X. Li, Z. Chen, P. Ruan, X. Hu, B. Lu et al., Inducing preferential growth of the Zn (002) plane by using a multifunctional chelator for achieving highly reversible Zn anodes. Nanoscale 16, 2923–2930 (2024). https://doi.org/10.1039/d3nr05699f
- J. Li, Z. Guo, J. Wu, Z. Zheng, Z. Yu et al., Dextran: a multifunctional and universal electrolyte additive for aqueous Zn ion batteries. Adv. Energy Mater. 13, 2301743 (2023). https://doi.org/10.1002/aenm.202301743
- L. Deng, X. Xie, W. Song, A. Pan, G. Cao et al., Realizing highly stable zinc anode via an electrolyte additive shield layer and electrochemical in-situ interface. Chem. Eng. J. 488, 151104 (2024). https://doi.org/10.1016/j.cej.2024.151104
- K. Wang, S. Zhang, X. Zhou, X. Yang, X. Li et al., Unambiguous discrimination of all 20 proteinogenic amino acids and their modifications by nanopore. Nat. Methods 21, 92–101 (2023). https://doi.org/10.1038/s41592-023-02021-8
- W. Kohn, L.J. Sham, Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965). https://doi.org/10.1103/PhysRev.140.A1133
- B. Hammer, L.B. Hansen, J.K. Norskov, Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1998). https://doi.org/10.1103/PhysRevB.59.7413
- B. Delley, From molecules to solids with the DMol3 approach. Chem. Phys. 113, 7756–7764 (2000). https://doi.org/10.1063/1.1316015
- Y. Inada, H. Orita, Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: evidence of small basis set superposition error compared to Gaussian basis sets. Comput. Chem. 29, 225–232 (2007). https://doi.org/10.1002/jcc.20782
- X. Zeng, Y. Liu, Y. Kang, Q. Li, Y. Xia et al., Simultaneously tuning charge separation and oxygen reduction pathway on graphitic carbon nitride by polyethylenimine for boosted photocatalytic hydrogen peroxide production. ACS Catal. 10, 3697–3706 (2020). https://doi.org/10.1021/acscatal.9b05247
- H. Sun, COMPASS: an ab initio force-field optimized for condensed-phase applications soverview with details on alkane and benzene compounds. J. Phys. Chem. B 102, 7338–7364 (1998). https://doi.org/10.1021/jp980939v
- D.-D. Zhou, P. Chen, C. Wang, S.-S. Wang, Y. Du et al., Intermediate-sized molecular sieving of styrene from larger and smaller analogues. Nat. Mater. 18, 994–998 (2019). https://doi.org/10.1038/s41563-019-0427-z
- E. Cauët, S. Bogatko, J.H. Weare, J.L. Fulton, G.K. Schenter et al., Structure and dynamics of the hydration shells of the Zn2+ ion from ab initio molecular dynamics and combined ab initio and classical molecular dynamics simulations. J. Chem. Phys. 132, 194502 (2010). https://doi.org/10.1063/1.3421542
- Y. Liu, X. Xu, M. Sadd, O.O. Kapitanova, V.A. Krivchenko et al., Insight into the critical role of exchange current density on electrodeposition behavior of lithium metal. Adv. Sci. 8, 2003301 (2021). https://doi.org/10.1002/advs.202003301
- S. Vajda, Technique of the analysis of variance. Nature 4053, 27 (1947). https://doi.org/10.1038/160027a0
- B. Li, P. Ruan, X. Xu, Z. He, X. Zhu et al., Covalent organic framework with 3D ordered channel and multi-functional groups endows Zn anode with superior stability. Nano-Micro Lett. 16, 76 (2024). https://doi.org/10.1007/s40820-023-01278-0
- Q. Zhao, W. Liu, X. Ni, H. Yu, C. Zhang et al., Steering interfacial renovation with highly electronegative Cl modulated trinity effect for exceptional durable zinc anode. Adv. Funct. Mater. (2024). https://doi.org/10.1002/adfm.202404219
- M. Wang, J. Ma, Y. Meng, J. Sun, Y. Yuan et al., High-capacity zinc anode with 96 % utilization rate enabled by solvation structure design. Angew. Chem. Int. Ed. 62, e202214966 (2023). https://doi.org/10.1002/anie.202214966
- M. Grechko, T. Hasegawa, F. D’Angelo, H. Ito, D. Turchinovich et al., Coupling between intra- and intermolecular motions in liquid water revealed by two-dimensional terahertz-infrared-visible spectroscopy. Nat. Commun. 9, 885 (2018). https://doi.org/10.1038/s41467-018-03303-y
- Y. Wu, Q. Hu, H. Liang, A. Wang, H. Xu et al., Electrostatic potential as solvent descriptor to enable rational electrolyte design for lithium batteries. Adv. Energy Mater. 13, 2300259 (2023). https://doi.org/10.1002/aenm.202300259
- B.-A. Mei, O. Munteshari, J. Lau, B. Dunn, L. Pilon, Physical interpretations of nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 122, 194–206 (2017). https://doi.org/10.1021/acs.jpcc.7b10582
- H. Ge, L. Qin, B. Zhang, L. Jiang, Y. Tang et al., An ionically crosslinked composite hydrogel electrolyte based on natural biomacromolecules for sustainable zinc-ion batteries. Nanoscale Horiz. (2024). https://doi.org/10.1039/d4nh00243a
- S. Karthika, T.K. Radhakrishnan, P. Kalaichelvi, A review of classical and nonclassical nucleation theories. Cryst. Growth Des. 16, 6663–6681 (2016). https://doi.org/10.1021/acs.cgd.6b00794
- J. Li, Z. Liu, S. Han, P. Zhou, B. Lu et al., Hetero nucleus growth stabilizing zinc anode for high-biosecurity zinc-ion batteries. Nano-Micro Lett. 15, 237 (2023). https://doi.org/10.1007/s40820-023-01206-2
- E. McCafferty, Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 47, 3202–3215 (2005). https://doi.org/10.1016/j.corsci.2005.05.046
- Z. Huang, Z. Li, Y. Wang, J. Cong, X. Wu et al., Regulating Zn(002) deposition toward long cycle life for Zn metal batteries. ACS Energy Lett. 8, 372–380 (2022). https://doi.org/10.1021/acsenergylett.2c02359
- R. Chen, W. Zhang, Q. Huang, C. Guan, W. Zong et al., Trace amounts of triple-functional additives enable reversible aqueous zinc-ion batteries from a comprehensive perspective. Nano-Micro Lett. 15, 143–154 (2023). https://doi.org/10.1007/s40820-023-01050-4
- K. Wu, J. Yi, X. Liu, Y. Sun, J. Cui et al., Regulating Zn deposition via an artificial solid–electrolyte interface with aligned dipoles for long life Zn anode. Nano-Micro Lett. 13, 79 (2021). https://doi.org/10.1007/s40820-021-00599-2
References
F. Wang, J. Xie, D. Zheng, F. Yang, H. Zhang et al., Intrinsic carbon defects induced reversible antimony chemistry for high-energy aqueous alkaline batteries. Adv. Mater. 34, 2200085 (2022). https://doi.org/10.1002/adma.202200085
D. Li, Y. Guo, C. Zhang, X. Chen, W. Zhang et al., Unveiling organic electrode materials in aqueous zinc-ion batteries: from structural design to electrochemical performance. Nano-Micro Lett. 16, 194 (2024). https://doi.org/10.1007/s40820-024-01404-6
W. Li, Z. Ji, F. Dong, Y. Yang, Evaluation of provincial renewable energy generation efficiency and spatio-temporal heterogeneity of influencing factors in China. Renew. Energy 226, 120446 (2024). https://doi.org/10.1016/j.renene.2024.120446
Y. Huang, Q. Gu, Z. Guo, W. Liu, Z. Chang et al., Unraveling dynamical behaviors of zinc metal electrodes in aqueous electrolytes through an operando study. Energy Storage Mater. 46, 243–251 (2022). https://doi.org/10.1016/j.ensm.2022.01.012
M. Han, D. Chen, Q. Lu, G. Fang, Aqueous rechargeable Zn–iodine batteries: issues, strategies and perspectives. Small 20, 2310293 (2023). https://doi.org/10.1002/smll.202310293
A. Zhou, H. Wang, F. Zhang, X. Hu, Z. Song et al., Amphipathic phenylalanine-induced nucleophilic-hydrophobic interface toward highly reversible Zn anode. Nano-Micro Lett. 16, 164 (2024). https://doi.org/10.1007/s40820-024-01380-x
X. Xie, J. Li, Z. Xing, B. Lu, S. Liang et al., Biocompatible zinc battery with programmable electro-cross-linked electrolyte. Natl. Sci. Rev. 10, nwac281 (2023). https://doi.org/10.1093/nsr/nwac281
X. Chen, X. Xie, P. Ruan, S. Liang, W.-Y. Wong et al., Thermodynamics and kinetics of conversion reaction in zinc batteries. ACS Energy Lett. 9, 2037–2056 (2024). https://doi.org/10.1021/acsenergylett.4c00450
M. Zhang, W. Xu, X. Han, H. Fan, T. Chen et al., Unveiling the mechanism of the dendrite nucleation and growth in aqueous zinc ion batteries. Adv. Energy Mater. 14, 2303737 (2023). https://doi.org/10.1002/aenm.202303737
Q. Zou, Z. Liang, W. Wang, D. Dong, Y.-C. Lu, A nuclei-rich strategy for highly reversible dendrite-free zinc metal anodes. Energy Environ. Sci. 16, 6026–6034 (2023). https://doi.org/10.1039/d3ee03246a
Z. Xiang, Y. Qiu, X. Guo, K. Qi, Z.-L. Xu et al., Inherited construction of porous zinc hydroxide sulfate layer for stable dendrite-free Zn anode. Energy Environ. Sci. 17, 3409–3418 (2024). https://doi.org/10.1039/d4ee00721b
Z. Khan, D. Kumar, X. Crispin, Does water-in-salt electrolyte subdue issues of Zn batteries? Adv. Mater. 35, 2300369 (2023). https://doi.org/10.1002/adma.202300369
Y. Liu, H. He, A. Gao, J. Ling, F. Yi et al., Fundamental study on Zn corrosion and dendrite growth in gel electrolyte towards advanced wearable Zn-ion battery. Chem. Eng. J. 446, 137021 (2022). https://doi.org/10.1016/j.cej.2022.137021
C. Huang, X. Zhao, Y. Hao, Y. Yang, Y. Qian et al., Selection criteria for electrical double layer structure regulators enabling stable Zn metal anodes. Energy Environ. Sci. 16, 1721–1731 (2023). https://doi.org/10.1039/d3ee00045a
T.C. Li, C. Lin, M. Luo, P. Wang, D.-S. Li et al., Interfacial molecule engineering for reversible Zn electrochemistry. ACS Energy Lett. 8, 3258–3268 (2023). https://doi.org/10.1021/acsenergylett.3c00859
J. Wu, Understanding the electric double-layer structure, capacitance, and charging dynamics. Chem. Rev. 122, 10821–10859 (2022). https://doi.org/10.1021/acs.chemrev.2c00097
W. Nie, H. Cheng, Q. Sun, S. Liang, X. Lu et al., Design strategies toward high-performance Zn metal anode. Small Methods 8, e2201572 (2024). https://doi.org/10.1002/smtd.202201572
T. Wang, Y. Tang, M. Yu, B. Lu, X. Zhang et al., Spirally grown zinc-cobalt alloy layer enables highly reversible zinc metal anodes. Adv. Funct. Mater. 33, 2306101 (2023). https://doi.org/10.1002/adfm.202306101
Y. Zhu, G. Liang, X. Cui, X. Liu, H. Zhong et al., Engineering hosts for Zn anodes in aqueous Zn-ion batteries. Energy Environ. Sci. 17, 369–385 (2024). https://doi.org/10.1039/d3ee03584k
J. Chen, H. Zhang, M. Fang, C. Ke, S. Liu et al., Design of localized high-concentration electrolytes via donor number. ACS Energy Lett. 8, 1723–1734 (2023). https://doi.org/10.1021/acsenergylett.3c00004
Y. Xu, X. Zhou, Z. Chen, Y. Hou, Y. You et al., Electrolyte formulas of aqueous zinc ion battery: a physical difference with chemical consequences. Mater. Today 66, 339–347 (2023). https://doi.org/10.1016/j.mattod.2023.04.005
M. Han, T.C. Li, X. Chen, H.Y. Yang, Electrolyte modulation strategies for low-temperature Zn batteries. Small 20, 2304901 (2023). https://doi.org/10.1002/smll.202304901
J. Weng, W. Zhu, K. Yu, J. Luo, M. Chen et al., Enhancing Zn-metal anode stability: key effects of electrolyte additives on ion-shield-like electrical double layer and stable solid electrolyte interphase. Adv. Funct. Mater. 34, 2314347 (2024). https://doi.org/10.1002/adfm.202314347
Y. Liu, B. Xie, Q. Hu, R. Zhao, Q. Zheng et al., Regulating the helmholtz plane by trace polarity additive for long-life Zn ion batteries. Energy Storage Mater. 66, 103202 (2024). https://doi.org/10.1016/j.ensm.2024.103202
X. Li, M. Wang, Y. Chu, Y. Gao, Z. Yang et al., Modulation of water reactivity by ethyl acetate/water co-solvent for zinc-metal batteries. Chem. Eng. J. 487, 150588 (2024). https://doi.org/10.1016/j.cej.2024.150588
Y. Yang, Y. Li, Q. Zhu, B. Xu, Optimal molecular configuration of electrolyte additives enabling stabilization of zinc anodes. Adv. Funct. Mater. (2024). https://doi.org/10.1002/adfm.202316371
Q. Meng, Q. Bai, R. Zhao, P. Cao, G. Zhang et al., Attenuating water activity through impeded proton transfer resulting from hydrogen bond enhancement effect for fast and ultra-stable Zn metal anode. Adv. Energy Mater. 13, 2302828 (2023). https://doi.org/10.1002/aenm.202302828
X. Li, Z. Chen, P. Ruan, X. Hu, B. Lu et al., Inducing preferential growth of the Zn (002) plane by using a multifunctional chelator for achieving highly reversible Zn anodes. Nanoscale 16, 2923–2930 (2024). https://doi.org/10.1039/d3nr05699f
J. Li, Z. Guo, J. Wu, Z. Zheng, Z. Yu et al., Dextran: a multifunctional and universal electrolyte additive for aqueous Zn ion batteries. Adv. Energy Mater. 13, 2301743 (2023). https://doi.org/10.1002/aenm.202301743
L. Deng, X. Xie, W. Song, A. Pan, G. Cao et al., Realizing highly stable zinc anode via an electrolyte additive shield layer and electrochemical in-situ interface. Chem. Eng. J. 488, 151104 (2024). https://doi.org/10.1016/j.cej.2024.151104
K. Wang, S. Zhang, X. Zhou, X. Yang, X. Li et al., Unambiguous discrimination of all 20 proteinogenic amino acids and their modifications by nanopore. Nat. Methods 21, 92–101 (2023). https://doi.org/10.1038/s41592-023-02021-8
W. Kohn, L.J. Sham, Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965). https://doi.org/10.1103/PhysRev.140.A1133
B. Hammer, L.B. Hansen, J.K. Norskov, Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1998). https://doi.org/10.1103/PhysRevB.59.7413
B. Delley, From molecules to solids with the DMol3 approach. Chem. Phys. 113, 7756–7764 (2000). https://doi.org/10.1063/1.1316015
Y. Inada, H. Orita, Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: evidence of small basis set superposition error compared to Gaussian basis sets. Comput. Chem. 29, 225–232 (2007). https://doi.org/10.1002/jcc.20782
X. Zeng, Y. Liu, Y. Kang, Q. Li, Y. Xia et al., Simultaneously tuning charge separation and oxygen reduction pathway on graphitic carbon nitride by polyethylenimine for boosted photocatalytic hydrogen peroxide production. ACS Catal. 10, 3697–3706 (2020). https://doi.org/10.1021/acscatal.9b05247
H. Sun, COMPASS: an ab initio force-field optimized for condensed-phase applications soverview with details on alkane and benzene compounds. J. Phys. Chem. B 102, 7338–7364 (1998). https://doi.org/10.1021/jp980939v
D.-D. Zhou, P. Chen, C. Wang, S.-S. Wang, Y. Du et al., Intermediate-sized molecular sieving of styrene from larger and smaller analogues. Nat. Mater. 18, 994–998 (2019). https://doi.org/10.1038/s41563-019-0427-z
E. Cauët, S. Bogatko, J.H. Weare, J.L. Fulton, G.K. Schenter et al., Structure and dynamics of the hydration shells of the Zn2+ ion from ab initio molecular dynamics and combined ab initio and classical molecular dynamics simulations. J. Chem. Phys. 132, 194502 (2010). https://doi.org/10.1063/1.3421542
Y. Liu, X. Xu, M. Sadd, O.O. Kapitanova, V.A. Krivchenko et al., Insight into the critical role of exchange current density on electrodeposition behavior of lithium metal. Adv. Sci. 8, 2003301 (2021). https://doi.org/10.1002/advs.202003301
S. Vajda, Technique of the analysis of variance. Nature 4053, 27 (1947). https://doi.org/10.1038/160027a0
B. Li, P. Ruan, X. Xu, Z. He, X. Zhu et al., Covalent organic framework with 3D ordered channel and multi-functional groups endows Zn anode with superior stability. Nano-Micro Lett. 16, 76 (2024). https://doi.org/10.1007/s40820-023-01278-0
Q. Zhao, W. Liu, X. Ni, H. Yu, C. Zhang et al., Steering interfacial renovation with highly electronegative Cl modulated trinity effect for exceptional durable zinc anode. Adv. Funct. Mater. (2024). https://doi.org/10.1002/adfm.202404219
M. Wang, J. Ma, Y. Meng, J. Sun, Y. Yuan et al., High-capacity zinc anode with 96 % utilization rate enabled by solvation structure design. Angew. Chem. Int. Ed. 62, e202214966 (2023). https://doi.org/10.1002/anie.202214966
M. Grechko, T. Hasegawa, F. D’Angelo, H. Ito, D. Turchinovich et al., Coupling between intra- and intermolecular motions in liquid water revealed by two-dimensional terahertz-infrared-visible spectroscopy. Nat. Commun. 9, 885 (2018). https://doi.org/10.1038/s41467-018-03303-y
Y. Wu, Q. Hu, H. Liang, A. Wang, H. Xu et al., Electrostatic potential as solvent descriptor to enable rational electrolyte design for lithium batteries. Adv. Energy Mater. 13, 2300259 (2023). https://doi.org/10.1002/aenm.202300259
B.-A. Mei, O. Munteshari, J. Lau, B. Dunn, L. Pilon, Physical interpretations of nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 122, 194–206 (2017). https://doi.org/10.1021/acs.jpcc.7b10582
H. Ge, L. Qin, B. Zhang, L. Jiang, Y. Tang et al., An ionically crosslinked composite hydrogel electrolyte based on natural biomacromolecules for sustainable zinc-ion batteries. Nanoscale Horiz. (2024). https://doi.org/10.1039/d4nh00243a
S. Karthika, T.K. Radhakrishnan, P. Kalaichelvi, A review of classical and nonclassical nucleation theories. Cryst. Growth Des. 16, 6663–6681 (2016). https://doi.org/10.1021/acs.cgd.6b00794
J. Li, Z. Liu, S. Han, P. Zhou, B. Lu et al., Hetero nucleus growth stabilizing zinc anode for high-biosecurity zinc-ion batteries. Nano-Micro Lett. 15, 237 (2023). https://doi.org/10.1007/s40820-023-01206-2
E. McCafferty, Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 47, 3202–3215 (2005). https://doi.org/10.1016/j.corsci.2005.05.046
Z. Huang, Z. Li, Y. Wang, J. Cong, X. Wu et al., Regulating Zn(002) deposition toward long cycle life for Zn metal batteries. ACS Energy Lett. 8, 372–380 (2022). https://doi.org/10.1021/acsenergylett.2c02359
R. Chen, W. Zhang, Q. Huang, C. Guan, W. Zong et al., Trace amounts of triple-functional additives enable reversible aqueous zinc-ion batteries from a comprehensive perspective. Nano-Micro Lett. 15, 143–154 (2023). https://doi.org/10.1007/s40820-023-01050-4
K. Wu, J. Yi, X. Liu, Y. Sun, J. Cui et al., Regulating Zn deposition via an artificial solid–electrolyte interface with aligned dipoles for long life Zn anode. Nano-Micro Lett. 13, 79 (2021). https://doi.org/10.1007/s40820-021-00599-2