Thioacetamide Additive Homogenizing Zn Deposition Revealed by In Situ Digital Holography for Advanced Zn Ion Batteries
Corresponding Author: Chao Wang
Nano-Micro Letters,
Vol. 16 (2024), Article Number: 117
Abstract
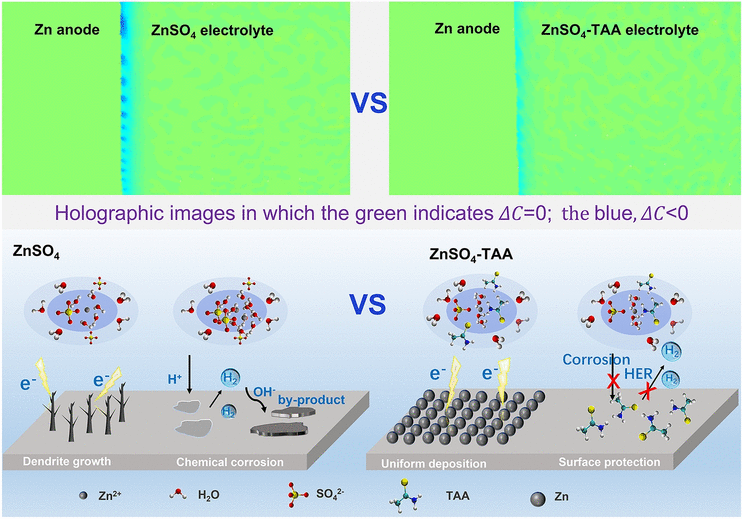
Zinc ion batteries are considered as potential energy storage devices due to their advantages of low-cost, high-safety, and high theoretical capacity. However, dendrite growth and chemical corrosion occurring on Zn anode limit their commercialization. These problems can be tackled through the optimization of the electrolyte. However, the screening of electrolyte additives using normal electrochemical methods is time-consuming and labor-intensive. Herein, a fast and simple method based on the digital holography is developed. It can realize the in situ monitoring of electrode/electrolyte interface and provide direct information concerning ion concentration evolution of the diffusion layer. It is effective and time-saving in estimating the homogeneity of the deposition layer and predicting the tendency of dendrite growth, thus able to value the applicability of electrolyte additives. The feasibility of this method is further validated by the forecast and evaluation of thioacetamide additive. Based on systematic characterization, it is proved that the introduction of thioacetamide can not only regulate the interficial ion flux to induce dendrite-free Zn deposition, but also construct adsorption molecule layers to inhibit side reactions of Zn anode. Being easy to operate, capable of in situ observation, and able to endure harsh conditions, digital holography method will be a promising approach for the interfacial investigation of other battery systems.
Highlights:
1 Digital holography can realize the in situ observation of electrode/electrolyte interface and provide dynamic evolution information of the liquid phase of electrode, which is both efficient and effective in investing the interficial electrochemical mechanism and screening electrolyte additives.
2 Thioacetamide electrolyte additive effectively enhances the electrochemical performance of Zn anode by regulating the interficial ion flux to induce dendrite-free Zn deposition and constructing adsorption molecule layers to inhibit side reactions.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Z. Zhu, T. Jiang, M. Ali, Y. Meng, Y. Jin et al., Rechargeable batteries for grid scale energy storage. Chem. Rev. 122, 16610–16751 (2022). https://doi.org/10.1021/acs.chemrev.2c00289
- J. Yang, B. Yin, Y. Sun, H. Pan, W. Sun et al., Zinc anode for mild aqueous zinc-ion batteries: challenges, strategies, and perspectives. Nano-Micro Lett. 14, 42 (2022). https://doi.org/10.1007/s40820-021-00782-5
- Y. Meng, M. Wang, J. Xu, K. Xu, K. Zhang et al., Balancing interfacial reactions through regulating p-band centers by an indium tin oxide protective layer for stable Zn metal anodes. Angew. Chem. Int. Ed. 62, e202308454 (2023). https://doi.org/10.1002/anie.202308454
- J.Y. Kim, G. Liu, R.E.A. Ardhi, J. Park, H. Kim et al., Stable Zn metal anodes with limited Zn-doping in MgF2 interphase for fast and uniformly ionic flux. Nano-Micro Lett. 14, 46 (2022). https://doi.org/10.1007/s40820-021-00788-z
- G. Ma, L. Miao, W. Yuan, K. Qiu, M. Liu et al., Non-flammable, dilute, and hydrous organic electrolytes for reversible Zn batteries. Chem. Sci. 13, 11320–11329 (2022). https://doi.org/10.1039/D2SC04143J
- X. Zheng, Z. Liu, J. Sun, R. Luo, K. Xu et al., Constructing robust heterostructured interface for anode-free zinc batteries with ultrahigh capacities. Nat. Commun. 14, 76 (2023). https://doi.org/10.1038/s41467-022-35630-6
- D. Wang, Q. Li, Y. Zhao, H. Hong, H. Li et al., Insight on organic molecules in aqueous Zn-ion batteries with an emphasis on the Zn anode regulation. Adv. Energy Mater. 12, 2102707 (2022). https://doi.org/10.1002/aenm.202102707
- W. Yuan, X. Nie, G. Ma, M. Liu, Y. Wang et al., Realizing textured zinc metal anodes through regulating electrodeposition current for aqueous zinc batteries. Angew. Chem. Int. Ed. 62, e202218386 (2023). https://doi.org/10.1002/anie.202218386
- M. Wang, J. Ma, Y. Meng, J. Sun, Y. Yuan et al., High-capacity zinc anode with 96 % utilization rate enabled by solvation structure design. Angew. Chem. Int. Ed. 62, e202214966 (2023). https://doi.org/10.1002/anie.202214966
- F. Wan, Y. Zhang, L. Zhang, D. Liu, C. Wang et al., Reversible oxygen redox chemistry in aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 58, 7062–7067 (2019). https://doi.org/10.1002/anie.201902679
- A. Guerfi, J. Trottier, I. Boyano, I. De Meatza, J.A. Blazquez et al., High cycling stability of zinc-anode/conducting polymer rechargeable battery with non-aqueous electrolyte. J. Power Sour. 248, 1099–1104 (2014). https://doi.org/10.1016/j.jpowsour.2013.09.082
- Q. Zhang, J. Luan, Y. Tang, X. Ji, H. Wang, Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 59, 13180–13191 (2020). https://doi.org/10.1002/anie.202000162
- R. Xu, X.-B. Cheng, C. Yan, X.-Q. Zhang, Y. Xiao et al., Artificial interphases for highly stable lithium metal anode. Matter 1, 317–344 (2019). https://doi.org/10.1016/j.matt.2019.05.016
- J. Ding, Z. Du, L. Gu, B. Li, L. Wang et al., Ultrafast Zn2+ intercalation and deintercalation in vanadium dioxide. Adv. Mater. 30, e1800762 (2018). https://doi.org/10.1002/adma.201800762
- F. Xie, L. Zhang, B. Chen, D. Chao, Q. Gu et al., Revealing the origin of improved reversible capacity of dual-shell bismuth boxes anode for potassium-ion batteries. Matter 1, 1681–1693 (2019). https://doi.org/10.1016/j.matt.2019.07.006
- Y. Zhang, Y. Liang, H. Dong, X. Wang, Y. Yao, Charge storage mechanism of a quinone polymer electrode for zinc-ion batteries. J. Electrochem. Soc. 167, 070558 (2020). https://doi.org/10.1149/1945-7111/ab847a
- S. Zhang, S. Long, H. Li, Q. Xu, A high-capacity organic cathode based on active N atoms for aqueous zinc-ion batteries. Chem. Eng. J. 400, 125898 (2020). https://doi.org/10.1016/j.cej.2020.125898
- P. He, G. Zhang, X. Liao, M. Yan, X. Xu et al., Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy Mater. 8, 1702463 (2018). https://doi.org/10.1002/aenm.201702463
- M.S. Javed, H. Lei, Z. Wang, B.-T. Liu, X. Cai et al., 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 70, 104573 (2020). https://doi.org/10.1016/j.nanoen.2020.104573
- W. Li, K. Wang, S. Cheng, K. Jiang, A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Stor. Mater. 15, 14–21 (2018). https://doi.org/10.1016/j.ensm.2018.03.003
- Z. Cao, J. Fu, M. Wu, T. Hua, H. Hu, Synchronously manipulating Zn2+ transfer and hydrogen/oxygen evolution kinetics in MXene host electrodes toward symmetric Zn-ions micro-supercapacitor with enhanced areal energy density. Energy Stor. Mater. 40, 10–21 (2021). https://doi.org/10.1016/j.ensm.2021.04.047
- Q. Lei, J. Zhang, Z. Liang, Y. Yue, Z. Ren et al., Synergistic engineering of sulfur vacancies and heterointerfaces in copper sulfide anodes for aqueous Zn-ion batteries with fast diffusion kinetics and an ultralong lifespan. Adv. Energy Mater. 12, 2200547 (2022). https://doi.org/10.1002/aenm.202200547
- Z. Yang, X. Pan, Y. Shen, R. Chen, T. Li et al., New insights into phase-mechanism relationship of Mgx MnO2 nanowires in aqueous zinc-ion batteries. Small 18, e2107743 (2022). https://doi.org/10.1002/smll.202107743
- R.M. Fernandez-Domene, R. Sánchez-Tovar, J. García-Antón, Passive behavior and passivity breakdown of AISI 304 in LiBr solutions through scanning electrochemical microscopy. J. Electrochem. Soc. 161, C565–C572 (2014). https://doi.org/10.1149/2.1051412jes
- B. Kinzer, A.L. Davis, T. Krauskopf, H. Hartmann, W.S. LePage et al., Operando analysis of the molten Li|LLZO interface: understanding how the physical properties of Li affect the critical current density. Matter 4, 1947–1961 (2021). https://doi.org/10.1016/j.matt.2021.04.016
- R.F. Schaller, A. Mishra, J.M. Rodelas, J.M. Taylor, E.J. Schindelholz, The role of microstructure and surface finish on the corrosion of selective laser melted 304L. J. Electrochem. Soc. 165, C234–C242 (2018). https://doi.org/10.1149/2.0431805jes
- Y. Yuan, K. Amine, J. Lu, R. Shahbazian-Yassar, Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 8, 15806 (2017). https://doi.org/10.1038/ncomms15806
- Q. Zhang, J. Ma, L. Mei, J. Liu, Z. Li et al., In situ TEM visualization of LiF nanosheet formation on the cathode-electrolyte interphase (CEI) in liquid-electrolyte lithium-ion batteries. Matter 5, 1235–1250 (2022). https://doi.org/10.1016/j.matt.2022.01.015
- S. Lee, I. Kang, J. Kim, S.H. Kim, K. Kang et al., Real-time visualization of Zn metal plating/stripping in aqueous batteries with high areal capacities. J. Power. Sour. 472, 228334 (2020). https://doi.org/10.1016/j.jpowsour.2020.228334
- D.A. Shapiro, Y.-S. Yu, T. Tyliszczak, J. Cabana, R. Celestre et al., Chemical composition mapping with nanometre resolution by soft X-ray microscopy. Nat. Photon. 8, 765–769 (2014). https://doi.org/10.1038/nphoton.2014.207
- N.-W. Li, Y. Shi, Y.-X. Yin, X.-X. Zeng, J.-Y. Li et al., A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Ed. 57, 1505–1509 (2018). https://doi.org/10.1002/anie.201710806
- S.-Y. Lang, R.-J. Xiao, L. Gu, Y.-G. Guo, R. Wen et al., Interfacial mechanism in lithium-sulfur batteries: how salts mediate the structure evolution and dynamics. J. Am. Chem. Soc. 140, 8147–8155 (2018). https://doi.org/10.1021/jacs.8b02057
- A. Miki, K. Nishikawa, T. Ozawa, H. Matsushima, M. Ueda, In situ measurement of Al3+ concentration profile during Al anodization using digital holographic interferometric microscope. J. Electrochem. Soc. 167, 062501 (2020). https://doi.org/10.1149/1945-7111/ab7bd6
- I. Arise, Y. Fukunaka, F.R. McLarnon, T. Abe, In situ observation at the surface of zinc in alkaline solution under pulsed current by holographic interferometry. J. Electrochem. Soc. 168, 080509 (2021). https://doi.org/10.1149/1945-7111/ac18e3
- P. Marquet, B. Rappaz, P.J. Magistretti, E. Cuche, Y. Emery et al., Digital holographic microscopy: a noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy. Opt. Lett. 30, 468–470 (2005). https://doi.org/10.1364/ol.30.000468
- Y. Wang, W. Jin, N. Ren, Dual-medium quantitative measurement simulation on cells. Appl. Opt. 50, 6440–6445 (2011). https://doi.org/10.1364/AO.50.006440
- L. Li, C. Wang, B. Yuan, S. Chen, Numerical reconstruction of digital holograms for the study of pitting dynamic processes of the X70 carbon steel in NaCl solution. Electrochem. Commun. 10, 103–107 (2008). https://doi.org/10.1016/j.elecom.2007.11.004
- X. Li, M. Zhang, B. Yuan, L. Li, C. Wang, Effects of the magnetic field on the corrosion dissolution of the 304 SS│FeCl3 system. Electrochim. Acta 222, 619–626 (2016). https://doi.org/10.1016/j.electacta.2016.11.017
- C. Lai, B. Yuan, H. Dai, K. Xi, C.J. Harris et al., Online digital holographic method for interface reaction monitoring in lithium-ion batteries. J. Phys. Chem. C 121, 24733–24739 (2017). https://doi.org/10.1021/acs.jpcc.7b09920
- H. Dai, B. Yuan, C. Bai, C. Lai, C. Wang, Communication—direct observation of the shuttle phenomenon in lithium-sulfur batteries via the digital holographic method. J. Electrochem. Soc. 165, A2866–A2868 (2018). https://doi.org/10.1149/2.1271811jes
- T.D. Kühne, M. Iannuzzi, M. Del Ben, V.V. Rybkin, P. Seewald et al., CP2K: an electronic structure and molecular dynamics software package-Quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020). https://doi.org/10.1063/5.0007045
- F. Neese, The orca program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. (2017). https://doi.org/10.1002/wcms.1327
- T. Lu, F. Chen, Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). https://doi.org/10.1002/jcc.22885
- J. Zhang, T. Lu, Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 23, 20323–20328 (2021). https://doi.org/10.1039/d1cp02805g
- W. Humphrey, A. Dalke, K. Schulten, VMD: visual molecular dynamics. J. Mol. Graph. 14(33–38), 27–28 (1996). https://doi.org/10.1016/0263-7855(96)00018-5
- M.J. Abraham, T. Murtola, R. Schulz, S. Páll, J.C. Smith et al., GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015). https://doi.org/10.1016/j.softx.2015.06.001
- H.J.C. Berendsen, D. van der Spoel, R. van Drunen, GROMACS: a message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995). https://doi.org/10.1016/0010-4655(95)00042-e
- Z. Cai, J. Wang, Z. Lu, R. Zhan, Y. Ou et al., Ultrafast metal electrodeposition revealed by in situ optical imaging and theoretical modeling toward fast-charging Zn battery chemistry. Angew. Chem. Int. Ed. 61, e202116560 (2022). https://doi.org/10.1002/anie.202116560
- H. Liu, Y. Zhang, C. Wang, J.N. Glazer, Z. Shan et al., Understanding and controlling the nucleation and growth of Zn electrodeposits for aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 13, 32930–32936 (2021). https://doi.org/10.1021/acsami.1c06131
- Z. Zhao, J. Zhao, Z. Hu, J. Li, J. Li et al., Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 12, 1938–1949 (2019). https://doi.org/10.1039/C9EE00596J
- L. Cao, D. Li, E. Hu, J. Xu, T. Deng et al., Solvation structure design for aqueous Zn metal batteries. J. Am. Chem. Soc. 142, 21404–21409 (2020). https://doi.org/10.1021/jacs.0c09794
- Q. Li, A. Chen, D. Wang, Y. Zhao, X. Wang et al., Tailoring the metal electrode morphology via electrochemical protocol optimization for long-lasting aqueous zinc batteries. Nat. Commun. 13, 3699 (2022). https://doi.org/10.1038/s41467-022-31461-7
- J. Li, L. He, F. Qin, J. Fang, B. Hong et al., Dual-enhancement on electrochemical performance with thioacetamide as an electrolyte additive for lithium-sulfur batteries. Electrochim. Acta 376, 138041 (2021). https://doi.org/10.1016/j.electacta.2021.138041
- H. Yu, D. Chen, Q. Li, C. Yan, Z. Jiang et al., In situ construction of anode–molecule interface via lone-pair electrons in trace organic molecules additives to achieve stable zinc metal anodes. Adv. Energy Mater. 13, 2300550 (2023). https://doi.org/10.1002/aenm.202300550
References
Z. Zhu, T. Jiang, M. Ali, Y. Meng, Y. Jin et al., Rechargeable batteries for grid scale energy storage. Chem. Rev. 122, 16610–16751 (2022). https://doi.org/10.1021/acs.chemrev.2c00289
J. Yang, B. Yin, Y. Sun, H. Pan, W. Sun et al., Zinc anode for mild aqueous zinc-ion batteries: challenges, strategies, and perspectives. Nano-Micro Lett. 14, 42 (2022). https://doi.org/10.1007/s40820-021-00782-5
Y. Meng, M. Wang, J. Xu, K. Xu, K. Zhang et al., Balancing interfacial reactions through regulating p-band centers by an indium tin oxide protective layer for stable Zn metal anodes. Angew. Chem. Int. Ed. 62, e202308454 (2023). https://doi.org/10.1002/anie.202308454
J.Y. Kim, G. Liu, R.E.A. Ardhi, J. Park, H. Kim et al., Stable Zn metal anodes with limited Zn-doping in MgF2 interphase for fast and uniformly ionic flux. Nano-Micro Lett. 14, 46 (2022). https://doi.org/10.1007/s40820-021-00788-z
G. Ma, L. Miao, W. Yuan, K. Qiu, M. Liu et al., Non-flammable, dilute, and hydrous organic electrolytes for reversible Zn batteries. Chem. Sci. 13, 11320–11329 (2022). https://doi.org/10.1039/D2SC04143J
X. Zheng, Z. Liu, J. Sun, R. Luo, K. Xu et al., Constructing robust heterostructured interface for anode-free zinc batteries with ultrahigh capacities. Nat. Commun. 14, 76 (2023). https://doi.org/10.1038/s41467-022-35630-6
D. Wang, Q. Li, Y. Zhao, H. Hong, H. Li et al., Insight on organic molecules in aqueous Zn-ion batteries with an emphasis on the Zn anode regulation. Adv. Energy Mater. 12, 2102707 (2022). https://doi.org/10.1002/aenm.202102707
W. Yuan, X. Nie, G. Ma, M. Liu, Y. Wang et al., Realizing textured zinc metal anodes through regulating electrodeposition current for aqueous zinc batteries. Angew. Chem. Int. Ed. 62, e202218386 (2023). https://doi.org/10.1002/anie.202218386
M. Wang, J. Ma, Y. Meng, J. Sun, Y. Yuan et al., High-capacity zinc anode with 96 % utilization rate enabled by solvation structure design. Angew. Chem. Int. Ed. 62, e202214966 (2023). https://doi.org/10.1002/anie.202214966
F. Wan, Y. Zhang, L. Zhang, D. Liu, C. Wang et al., Reversible oxygen redox chemistry in aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 58, 7062–7067 (2019). https://doi.org/10.1002/anie.201902679
A. Guerfi, J. Trottier, I. Boyano, I. De Meatza, J.A. Blazquez et al., High cycling stability of zinc-anode/conducting polymer rechargeable battery with non-aqueous electrolyte. J. Power Sour. 248, 1099–1104 (2014). https://doi.org/10.1016/j.jpowsour.2013.09.082
Q. Zhang, J. Luan, Y. Tang, X. Ji, H. Wang, Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 59, 13180–13191 (2020). https://doi.org/10.1002/anie.202000162
R. Xu, X.-B. Cheng, C. Yan, X.-Q. Zhang, Y. Xiao et al., Artificial interphases for highly stable lithium metal anode. Matter 1, 317–344 (2019). https://doi.org/10.1016/j.matt.2019.05.016
J. Ding, Z. Du, L. Gu, B. Li, L. Wang et al., Ultrafast Zn2+ intercalation and deintercalation in vanadium dioxide. Adv. Mater. 30, e1800762 (2018). https://doi.org/10.1002/adma.201800762
F. Xie, L. Zhang, B. Chen, D. Chao, Q. Gu et al., Revealing the origin of improved reversible capacity of dual-shell bismuth boxes anode for potassium-ion batteries. Matter 1, 1681–1693 (2019). https://doi.org/10.1016/j.matt.2019.07.006
Y. Zhang, Y. Liang, H. Dong, X. Wang, Y. Yao, Charge storage mechanism of a quinone polymer electrode for zinc-ion batteries. J. Electrochem. Soc. 167, 070558 (2020). https://doi.org/10.1149/1945-7111/ab847a
S. Zhang, S. Long, H. Li, Q. Xu, A high-capacity organic cathode based on active N atoms for aqueous zinc-ion batteries. Chem. Eng. J. 400, 125898 (2020). https://doi.org/10.1016/j.cej.2020.125898
P. He, G. Zhang, X. Liao, M. Yan, X. Xu et al., Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy Mater. 8, 1702463 (2018). https://doi.org/10.1002/aenm.201702463
M.S. Javed, H. Lei, Z. Wang, B.-T. Liu, X. Cai et al., 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 70, 104573 (2020). https://doi.org/10.1016/j.nanoen.2020.104573
W. Li, K. Wang, S. Cheng, K. Jiang, A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Stor. Mater. 15, 14–21 (2018). https://doi.org/10.1016/j.ensm.2018.03.003
Z. Cao, J. Fu, M. Wu, T. Hua, H. Hu, Synchronously manipulating Zn2+ transfer and hydrogen/oxygen evolution kinetics in MXene host electrodes toward symmetric Zn-ions micro-supercapacitor with enhanced areal energy density. Energy Stor. Mater. 40, 10–21 (2021). https://doi.org/10.1016/j.ensm.2021.04.047
Q. Lei, J. Zhang, Z. Liang, Y. Yue, Z. Ren et al., Synergistic engineering of sulfur vacancies and heterointerfaces in copper sulfide anodes for aqueous Zn-ion batteries with fast diffusion kinetics and an ultralong lifespan. Adv. Energy Mater. 12, 2200547 (2022). https://doi.org/10.1002/aenm.202200547
Z. Yang, X. Pan, Y. Shen, R. Chen, T. Li et al., New insights into phase-mechanism relationship of Mgx MnO2 nanowires in aqueous zinc-ion batteries. Small 18, e2107743 (2022). https://doi.org/10.1002/smll.202107743
R.M. Fernandez-Domene, R. Sánchez-Tovar, J. García-Antón, Passive behavior and passivity breakdown of AISI 304 in LiBr solutions through scanning electrochemical microscopy. J. Electrochem. Soc. 161, C565–C572 (2014). https://doi.org/10.1149/2.1051412jes
B. Kinzer, A.L. Davis, T. Krauskopf, H. Hartmann, W.S. LePage et al., Operando analysis of the molten Li|LLZO interface: understanding how the physical properties of Li affect the critical current density. Matter 4, 1947–1961 (2021). https://doi.org/10.1016/j.matt.2021.04.016
R.F. Schaller, A. Mishra, J.M. Rodelas, J.M. Taylor, E.J. Schindelholz, The role of microstructure and surface finish on the corrosion of selective laser melted 304L. J. Electrochem. Soc. 165, C234–C242 (2018). https://doi.org/10.1149/2.0431805jes
Y. Yuan, K. Amine, J. Lu, R. Shahbazian-Yassar, Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 8, 15806 (2017). https://doi.org/10.1038/ncomms15806
Q. Zhang, J. Ma, L. Mei, J. Liu, Z. Li et al., In situ TEM visualization of LiF nanosheet formation on the cathode-electrolyte interphase (CEI) in liquid-electrolyte lithium-ion batteries. Matter 5, 1235–1250 (2022). https://doi.org/10.1016/j.matt.2022.01.015
S. Lee, I. Kang, J. Kim, S.H. Kim, K. Kang et al., Real-time visualization of Zn metal plating/stripping in aqueous batteries with high areal capacities. J. Power. Sour. 472, 228334 (2020). https://doi.org/10.1016/j.jpowsour.2020.228334
D.A. Shapiro, Y.-S. Yu, T. Tyliszczak, J. Cabana, R. Celestre et al., Chemical composition mapping with nanometre resolution by soft X-ray microscopy. Nat. Photon. 8, 765–769 (2014). https://doi.org/10.1038/nphoton.2014.207
N.-W. Li, Y. Shi, Y.-X. Yin, X.-X. Zeng, J.-Y. Li et al., A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Ed. 57, 1505–1509 (2018). https://doi.org/10.1002/anie.201710806
S.-Y. Lang, R.-J. Xiao, L. Gu, Y.-G. Guo, R. Wen et al., Interfacial mechanism in lithium-sulfur batteries: how salts mediate the structure evolution and dynamics. J. Am. Chem. Soc. 140, 8147–8155 (2018). https://doi.org/10.1021/jacs.8b02057
A. Miki, K. Nishikawa, T. Ozawa, H. Matsushima, M. Ueda, In situ measurement of Al3+ concentration profile during Al anodization using digital holographic interferometric microscope. J. Electrochem. Soc. 167, 062501 (2020). https://doi.org/10.1149/1945-7111/ab7bd6
I. Arise, Y. Fukunaka, F.R. McLarnon, T. Abe, In situ observation at the surface of zinc in alkaline solution under pulsed current by holographic interferometry. J. Electrochem. Soc. 168, 080509 (2021). https://doi.org/10.1149/1945-7111/ac18e3
P. Marquet, B. Rappaz, P.J. Magistretti, E. Cuche, Y. Emery et al., Digital holographic microscopy: a noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy. Opt. Lett. 30, 468–470 (2005). https://doi.org/10.1364/ol.30.000468
Y. Wang, W. Jin, N. Ren, Dual-medium quantitative measurement simulation on cells. Appl. Opt. 50, 6440–6445 (2011). https://doi.org/10.1364/AO.50.006440
L. Li, C. Wang, B. Yuan, S. Chen, Numerical reconstruction of digital holograms for the study of pitting dynamic processes of the X70 carbon steel in NaCl solution. Electrochem. Commun. 10, 103–107 (2008). https://doi.org/10.1016/j.elecom.2007.11.004
X. Li, M. Zhang, B. Yuan, L. Li, C. Wang, Effects of the magnetic field on the corrosion dissolution of the 304 SS│FeCl3 system. Electrochim. Acta 222, 619–626 (2016). https://doi.org/10.1016/j.electacta.2016.11.017
C. Lai, B. Yuan, H. Dai, K. Xi, C.J. Harris et al., Online digital holographic method for interface reaction monitoring in lithium-ion batteries. J. Phys. Chem. C 121, 24733–24739 (2017). https://doi.org/10.1021/acs.jpcc.7b09920
H. Dai, B. Yuan, C. Bai, C. Lai, C. Wang, Communication—direct observation of the shuttle phenomenon in lithium-sulfur batteries via the digital holographic method. J. Electrochem. Soc. 165, A2866–A2868 (2018). https://doi.org/10.1149/2.1271811jes
T.D. Kühne, M. Iannuzzi, M. Del Ben, V.V. Rybkin, P. Seewald et al., CP2K: an electronic structure and molecular dynamics software package-Quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020). https://doi.org/10.1063/5.0007045
F. Neese, The orca program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. (2017). https://doi.org/10.1002/wcms.1327
T. Lu, F. Chen, Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). https://doi.org/10.1002/jcc.22885
J. Zhang, T. Lu, Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 23, 20323–20328 (2021). https://doi.org/10.1039/d1cp02805g
W. Humphrey, A. Dalke, K. Schulten, VMD: visual molecular dynamics. J. Mol. Graph. 14(33–38), 27–28 (1996). https://doi.org/10.1016/0263-7855(96)00018-5
M.J. Abraham, T. Murtola, R. Schulz, S. Páll, J.C. Smith et al., GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015). https://doi.org/10.1016/j.softx.2015.06.001
H.J.C. Berendsen, D. van der Spoel, R. van Drunen, GROMACS: a message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995). https://doi.org/10.1016/0010-4655(95)00042-e
Z. Cai, J. Wang, Z. Lu, R. Zhan, Y. Ou et al., Ultrafast metal electrodeposition revealed by in situ optical imaging and theoretical modeling toward fast-charging Zn battery chemistry. Angew. Chem. Int. Ed. 61, e202116560 (2022). https://doi.org/10.1002/anie.202116560
H. Liu, Y. Zhang, C. Wang, J.N. Glazer, Z. Shan et al., Understanding and controlling the nucleation and growth of Zn electrodeposits for aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 13, 32930–32936 (2021). https://doi.org/10.1021/acsami.1c06131
Z. Zhao, J. Zhao, Z. Hu, J. Li, J. Li et al., Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 12, 1938–1949 (2019). https://doi.org/10.1039/C9EE00596J
L. Cao, D. Li, E. Hu, J. Xu, T. Deng et al., Solvation structure design for aqueous Zn metal batteries. J. Am. Chem. Soc. 142, 21404–21409 (2020). https://doi.org/10.1021/jacs.0c09794
Q. Li, A. Chen, D. Wang, Y. Zhao, X. Wang et al., Tailoring the metal electrode morphology via electrochemical protocol optimization for long-lasting aqueous zinc batteries. Nat. Commun. 13, 3699 (2022). https://doi.org/10.1038/s41467-022-31461-7
J. Li, L. He, F. Qin, J. Fang, B. Hong et al., Dual-enhancement on electrochemical performance with thioacetamide as an electrolyte additive for lithium-sulfur batteries. Electrochim. Acta 376, 138041 (2021). https://doi.org/10.1016/j.electacta.2021.138041
H. Yu, D. Chen, Q. Li, C. Yan, Z. Jiang et al., In situ construction of anode–molecule interface via lone-pair electrons in trace organic molecules additives to achieve stable zinc metal anodes. Adv. Energy Mater. 13, 2300550 (2023). https://doi.org/10.1002/aenm.202300550