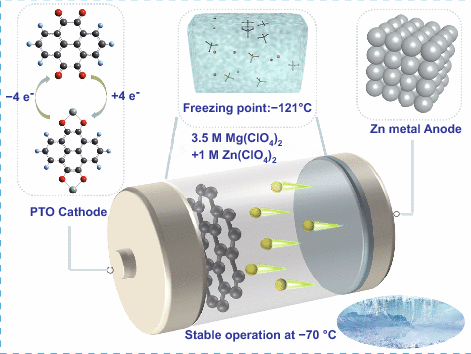
Synergistic Effect of Cation and Anion for Low-Temperature Aqueous Zinc-Ion Battery
Corresponding Author: Zhanliang Tao
Nano-Micro Letters,
Vol. 13 (2021), Article Number: 204
Abstract
Although aqueous zinc-ion batteries have gained great development due to their many merits, the frozen aqueous electrolyte hinders their practical application at low temperature conditions. Here, the synergistic effect of cation and anion to break the hydrogen-bonds network of original water molecules is demonstrated by multi-perspective characterization. Then, an aqueous-salt hydrates deep eutectic solvent of 3.5 M Mg(ClO4)2 + 1 M Zn(ClO4)2 is proposed and displays an ultralow freezing point of − 121 °C. A high ionic conductivity of 1.41 mS cm−1 and low viscosity of 22.9 mPa s at − 70 °C imply a fast ions transport behavior of this electrolyte. With the benefits of the low-temperature electrolyte, the fabricated Zn||Pyrene-4,5,9,10-tetraone (PTO) and Zn||Phenazine (PNZ) batteries exhibit satisfactory low-temperature performance. For example, Zn||PTO battery shows a high discharge capacity of 101.5 mAh g−1 at 0.5 C (200 mA g−1) and 71 mAh g−1 at 3 C (1.2 A g−1) when the temperature drops to − 70 °C. This work provides an unique view to design anti-freezing aqueous electrolyte.
Highlights:
1 The ratio of hydrogen bonds in water molecules is significantly decreased by introducing oxygen-ligand Mg2+ and hydrogen-ligand ClO4−, resulting in an ultralow solidifying point of − 121 °C.
2 The excellent low-temperature physicochemical properties and good compatibility with Zn metal of 3.5 M (mol L−1) Mg(ClO4)2 + 1 M Zn(ClO4)2 electrolyte gives fabricated Zn||pyrene-4,5,9,10-tetraone (PTO) battery and Zn||Phenazine (PNZ) battery a satisfactory low temperature performance.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Z. Tie, L. Liu, S. Deng, D. Zhao, Z. Niu, Proton insertion chemistry of a zinc-organic battery. Angew. Chem. Int. Ed. 59, 4920–4924 (2020). https://doi.org/10.1002/anie.201916529
- K. Zhu, T. Wu, K. Huang, NaCa0.6V6O16·3H2O as an ultra-stable cathode for Zn-ion batteries: the roles of pre-inserted dual-cations and structural water in V3O8 layer. Adv. Energy Mater. 9, 1901968 (2019). https://doi.org/10.1002/aenm.201901968
- X. Yuan, T. Sun, S. Zheng, J. Bao, J. Liang et al., An inverse-spinel Mg2MnO4 cathode for high-performance and flexible aqueous zinc-ion batteries. J. Mater. Chem. A 8, 22686–22693 (2020). https://doi.org/10.1039/d0ta08916h
- K.W. Nam, H. Kim, Y. Beldjoudi, T.W. Kwon, D.J. Kim et al., Redox-active phenanthrenequinone triangles in aqueous rechargeable zinc batteries. J. Am. Chem. Soc. 142, 2541–2548 (2020). https://doi.org/10.1021/jacs.9b12436
- J. Hao, X. Li, X. Zeng, D. Li, J. Mao et al., Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 13, 3917–3949 (2020). https://doi.org/10.1039/d0ee02162h
- J. Hao, J. Long, B. Li, X. Li, S. Zhang et al., Toward high-performance hybrid Zn-based batteries via deeply understanding their mechanism and using electrolyte additive. Adv. Funct. Mater. 29, 1903605 (2019). https://doi.org/10.1002/adfm.201903605
- C.X. Zhao, J.N. Liu, N. Yao, J. Wang, D. Ren et al., Can aqueous zinc-air batteries work at sub-zero temperatures? Angew. Chem. Int. Ed. 60, 15281–15285 (2021). https://doi.org/10.1002/anie.202104171
- Q. Zhang, K.X. Xia, Y.L. Ma, Y. Lu, L. Li et al., Chaotropic anion and fast-kinetics cathode enabling low-temperature aqueous Zn batteries. ACS Energy Lett. 6, 2704–2712 (2021). https://doi.org/10.1021/acsenergylett.1c01054
- Q. Zhang, Y.L. Ma, Y. Lu, L. Li, F. Wan et al., Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 11, 4463 (2020). https://doi.org/10.1038/s41467-020-18284-0
- T.J. Sun, H.H. Du, S.B. Zheng, J.Q. Shi, Z.L. Tao, High power and energy density aqueous proton battery operated at − 90 °C. Adv. Funct. Mater. 31, 2010127 (2021). https://doi.org/10.1002/adfm.202010127
- Q. Nian, J. Wang, S. Liu, T. Sun, S. Zheng et al., Aqueous batteries operated at − 50 °C. Angew. Chem. Int. Ed. 58, 16994–16999 (2019). https://doi.org/10.1002/anie.201908913
- J. Hao, L. Yuan, C. Ye, D. Chao, K. Davey et al., Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew. Chem. Int. Ed. 60, 7366–7375 (2021). https://doi.org/10.1002/anie.202016531
- M. Becker, R.S. Kuhnel, C. Battaglia, Water-in-salt electrolytes for aqueous lithium-ion batteries with liquidus temperatures below − 10 °C. Chem. Commun. 55, 12032–12035 (2019). https://doi.org/10.1039/c9cc04495g
- L. Jiang, Y. Lu, C. Zhao, L. Liu, J. Zhang et al., Building aqueous K-ion batteries for energy storage. Nat. Energy 4, 495–503 (2019). https://doi.org/10.1038/s41560-019-0388-0
- Z. Pei, Z. Yuan, C. Wang, S. Zhao, J. Fei et al., A flexible rechargeable zinc-air battery with excellent low-temperature adaptability. Angew. Chem. Int. Ed. 59, 4793–4799 (2020). https://doi.org/10.1002/anie.201915836
- C. Lu, X. Chen, All-temperature flexible supercapacitors enabled by antifreezing and thermally stable hydrogel electrolyte. Nano Lett. 20, 1907–1914 (2020). https://doi.org/10.1021/acs.nanolett.9b05148
- J.W. Zhao, J. Zhang, W.H. Yang, B.B. Chen, Z.M. Zhao et al., “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 57, 625–634 (2019). https://doi.org/10.1016/j.nanoen.2018.12.086
- W. Yang, X. Du, J. Zhao, Z. Chen, J. Li et al., Hydrated eutectic electrolytes with ligand-oriented solvation shells for long-cycling zinc-organic batteries. Joule 4, 1557–1574 (2020). https://doi.org/10.1016/j.joule.2020.05.018
- J. Shi, T. Sun, J. Bao, S. Zheng, H. Du et al., “Water-in-deep eutectic solvent” electrolytes for high-performance aqueous Zn-ion batteries. Adv. Funct. Mater. 31, 2102035 (2021). https://doi.org/10.1002/adfm.202102035
- X. Bu, Y. Zhang, Y. Sun, L. Su, J. Meng et al., All-climate aqueous supercapacitor enabled by a deep eutectic solvent electrolyte based on salt hydrate. J. Energy Chem. 49, 198–204 (2020). https://doi.org/10.1016/j.jechem.2020.02.042
- Y. Marcus, Unconventional deep eutectic solvents: aqueous salt hydrates. ACS Sustain. Chem. Eng. 5, 11780–11787 (2017). https://doi.org/10.1021/acssuschemeng.7b03528
- T. Sun, X. Yuan, K. Wang, S. Zheng, J. Shi et al., An ultralow-temperature aqueous zinc-ion battery. J. Mater. Chem. A 9, 7042–7047 (2021). https://doi.org/10.1039/d0ta12409e
- L. Cao, D. Li, F.A. Soto, V. Ponce, B. Zhang et al., Highly reversible aqueous zinc batteries enabled by zincophilic-zincophobic interfacial layers and interrupted hydrogen-bond electrolytes. Angew. Chem. Int. Ed. 60, 18845–18851 (2021). https://doi.org/10.1002/anie.202107378
- Y. Sun, H. Ma, X. Zhang, B. Liu, L. Liu et al., Salty ice electrolyte with superior ionic conductivity towards low-temperature aqueous zinc ion hybrid capacitors. Adv. Funct. Mater. 31, 2101277 (2021). https://doi.org/10.1002/adfm.202101277
- K. Nieszporek, J. Nieszporek, Multi-centred hydrogen bonds between water and perchlorate anion. Phys. Chem. Liq. 55, 473–481 (2016). https://doi.org/10.1080/00319104.2016.1227811
- K. Nieszporek, P. Podkoscielny, J. Nieszporek, Transitional hydrogen bonds in aqueous perchlorate solution. Phys. Chem. Chem. Phys. 18, 5957–5963 (2016). https://doi.org/10.1039/c5cp07831h
- B. Tansel, J. Sager, T. Rector, J. Garland, R.F. Strayer et al., Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 51, 40–47 (2006). https://doi.org/10.1016/j.seppur.2005.12.020
- F. David, V. Vokhminz, G. Ionova, Water characteristics depend on the ionic environment. Thermodynamics and modelisation of the aquo ions. J. Mol. Liq. 90, 45–62 (2001). https://doi.org/10.1016/S0167-7322(01)00106-4
- M. Śmiechowski, J. Stangret, ATR FT-IR H2O spectra of acidic aqueous solutions. Insights about proton hydration. J. Mol. Struct. 878, 104–115 (2008). https://doi.org/10.1016/j.molstruc.2007.08.001
- Y. Chen, Y.-H. Zhang, L.-J. Zhao, ATR-FTIR spectroscopic studies on aqueous LiClO4, NaClO4, and Mg(ClO4)2 solutions. Phys. Chem. Chem. Phys. 6, 537–542 (2004). https://doi.org/10.1039/b311768e
- Z. Dou, L. Wang, J. Hu, W. Fang, C. Sun et al., Hydrogen bonding effect on Raman modes of formic acid-water binary solutions. J. Mol. Liq. 313, 113595 (2020). https://doi.org/10.1016/j.molliq.2020.113595
- S.L. Simon, Temperature-modulated differential scanning calorimetry: theory and application. Thermochim. Acta 374, 55–71 (2001). https://doi.org/10.1016/S0040-6031(01)00493-2
- F. Wan, L. Zhang, X. Dai, X. Wang, Z. Niu et al., Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 9, 1656 (2018). https://doi.org/10.1038/s41467-018-04060-8
- M. Zhu, X. Wang, H. Tang, J. Wang, Q. Hao et al., Antifreezing hydrogel with high zinc reversibility for flexible and durable aqueous batteries by cooperative hydrated cations. Adv. Funct. Mater. 30, 1907218 (2019). https://doi.org/10.1002/adfm.201907218
- P. Wang, X. Xie, Z. Xing, X. Chen, G. Fang et al., Mechanistic insights of Mg2+-electrolyte additive for high-energy and long-life zinc-ion hybrid capacitors. Adv. Energy Mater. 11, 2102258 (2021). https://doi.org/10.1002/aenm.202101158
- T. Sun, C. Liu, X. Xu, Q. Nian, S. Zheng et al., Insights into the hydronium-ion storage of alloxazine in mild electrolyte. J. Mater. Chem. A 8, 21983–21987 (2020). https://doi.org/10.1039/d0ta09316e
- Z. Guo, J. Huang, X. Dong, Y. Xia, L. Yan et al., An organic/inorganic electrode-based hydronium-ion battery. Nat. Commun. 11, 959 (2020). https://doi.org/10.1038/s41467-020-14748-5
- T. Sun, C. Liu, J. Wang, Q. Nian, Y. Feng et al., A phenazine anode for high-performance aqueous rechargeable batteries in a wide temperature range. Nano Res. 13, 676–683 (2020). https://doi.org/10.1007/s12274-020-2674-3
- J. Hong, M. Lee, B. Lee, D.-H. Seo, C.B. Park et al., Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 5, 5335 (2014). https://doi.org/10.1038/ncomms6335
References
Z. Tie, L. Liu, S. Deng, D. Zhao, Z. Niu, Proton insertion chemistry of a zinc-organic battery. Angew. Chem. Int. Ed. 59, 4920–4924 (2020). https://doi.org/10.1002/anie.201916529
K. Zhu, T. Wu, K. Huang, NaCa0.6V6O16·3H2O as an ultra-stable cathode for Zn-ion batteries: the roles of pre-inserted dual-cations and structural water in V3O8 layer. Adv. Energy Mater. 9, 1901968 (2019). https://doi.org/10.1002/aenm.201901968
X. Yuan, T. Sun, S. Zheng, J. Bao, J. Liang et al., An inverse-spinel Mg2MnO4 cathode for high-performance and flexible aqueous zinc-ion batteries. J. Mater. Chem. A 8, 22686–22693 (2020). https://doi.org/10.1039/d0ta08916h
K.W. Nam, H. Kim, Y. Beldjoudi, T.W. Kwon, D.J. Kim et al., Redox-active phenanthrenequinone triangles in aqueous rechargeable zinc batteries. J. Am. Chem. Soc. 142, 2541–2548 (2020). https://doi.org/10.1021/jacs.9b12436
J. Hao, X. Li, X. Zeng, D. Li, J. Mao et al., Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 13, 3917–3949 (2020). https://doi.org/10.1039/d0ee02162h
J. Hao, J. Long, B. Li, X. Li, S. Zhang et al., Toward high-performance hybrid Zn-based batteries via deeply understanding their mechanism and using electrolyte additive. Adv. Funct. Mater. 29, 1903605 (2019). https://doi.org/10.1002/adfm.201903605
C.X. Zhao, J.N. Liu, N. Yao, J. Wang, D. Ren et al., Can aqueous zinc-air batteries work at sub-zero temperatures? Angew. Chem. Int. Ed. 60, 15281–15285 (2021). https://doi.org/10.1002/anie.202104171
Q. Zhang, K.X. Xia, Y.L. Ma, Y. Lu, L. Li et al., Chaotropic anion and fast-kinetics cathode enabling low-temperature aqueous Zn batteries. ACS Energy Lett. 6, 2704–2712 (2021). https://doi.org/10.1021/acsenergylett.1c01054
Q. Zhang, Y.L. Ma, Y. Lu, L. Li, F. Wan et al., Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 11, 4463 (2020). https://doi.org/10.1038/s41467-020-18284-0
T.J. Sun, H.H. Du, S.B. Zheng, J.Q. Shi, Z.L. Tao, High power and energy density aqueous proton battery operated at − 90 °C. Adv. Funct. Mater. 31, 2010127 (2021). https://doi.org/10.1002/adfm.202010127
Q. Nian, J. Wang, S. Liu, T. Sun, S. Zheng et al., Aqueous batteries operated at − 50 °C. Angew. Chem. Int. Ed. 58, 16994–16999 (2019). https://doi.org/10.1002/anie.201908913
J. Hao, L. Yuan, C. Ye, D. Chao, K. Davey et al., Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew. Chem. Int. Ed. 60, 7366–7375 (2021). https://doi.org/10.1002/anie.202016531
M. Becker, R.S. Kuhnel, C. Battaglia, Water-in-salt electrolytes for aqueous lithium-ion batteries with liquidus temperatures below − 10 °C. Chem. Commun. 55, 12032–12035 (2019). https://doi.org/10.1039/c9cc04495g
L. Jiang, Y. Lu, C. Zhao, L. Liu, J. Zhang et al., Building aqueous K-ion batteries for energy storage. Nat. Energy 4, 495–503 (2019). https://doi.org/10.1038/s41560-019-0388-0
Z. Pei, Z. Yuan, C. Wang, S. Zhao, J. Fei et al., A flexible rechargeable zinc-air battery with excellent low-temperature adaptability. Angew. Chem. Int. Ed. 59, 4793–4799 (2020). https://doi.org/10.1002/anie.201915836
C. Lu, X. Chen, All-temperature flexible supercapacitors enabled by antifreezing and thermally stable hydrogel electrolyte. Nano Lett. 20, 1907–1914 (2020). https://doi.org/10.1021/acs.nanolett.9b05148
J.W. Zhao, J. Zhang, W.H. Yang, B.B. Chen, Z.M. Zhao et al., “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 57, 625–634 (2019). https://doi.org/10.1016/j.nanoen.2018.12.086
W. Yang, X. Du, J. Zhao, Z. Chen, J. Li et al., Hydrated eutectic electrolytes with ligand-oriented solvation shells for long-cycling zinc-organic batteries. Joule 4, 1557–1574 (2020). https://doi.org/10.1016/j.joule.2020.05.018
J. Shi, T. Sun, J. Bao, S. Zheng, H. Du et al., “Water-in-deep eutectic solvent” electrolytes for high-performance aqueous Zn-ion batteries. Adv. Funct. Mater. 31, 2102035 (2021). https://doi.org/10.1002/adfm.202102035
X. Bu, Y. Zhang, Y. Sun, L. Su, J. Meng et al., All-climate aqueous supercapacitor enabled by a deep eutectic solvent electrolyte based on salt hydrate. J. Energy Chem. 49, 198–204 (2020). https://doi.org/10.1016/j.jechem.2020.02.042
Y. Marcus, Unconventional deep eutectic solvents: aqueous salt hydrates. ACS Sustain. Chem. Eng. 5, 11780–11787 (2017). https://doi.org/10.1021/acssuschemeng.7b03528
T. Sun, X. Yuan, K. Wang, S. Zheng, J. Shi et al., An ultralow-temperature aqueous zinc-ion battery. J. Mater. Chem. A 9, 7042–7047 (2021). https://doi.org/10.1039/d0ta12409e
L. Cao, D. Li, F.A. Soto, V. Ponce, B. Zhang et al., Highly reversible aqueous zinc batteries enabled by zincophilic-zincophobic interfacial layers and interrupted hydrogen-bond electrolytes. Angew. Chem. Int. Ed. 60, 18845–18851 (2021). https://doi.org/10.1002/anie.202107378
Y. Sun, H. Ma, X. Zhang, B. Liu, L. Liu et al., Salty ice electrolyte with superior ionic conductivity towards low-temperature aqueous zinc ion hybrid capacitors. Adv. Funct. Mater. 31, 2101277 (2021). https://doi.org/10.1002/adfm.202101277
K. Nieszporek, J. Nieszporek, Multi-centred hydrogen bonds between water and perchlorate anion. Phys. Chem. Liq. 55, 473–481 (2016). https://doi.org/10.1080/00319104.2016.1227811
K. Nieszporek, P. Podkoscielny, J. Nieszporek, Transitional hydrogen bonds in aqueous perchlorate solution. Phys. Chem. Chem. Phys. 18, 5957–5963 (2016). https://doi.org/10.1039/c5cp07831h
B. Tansel, J. Sager, T. Rector, J. Garland, R.F. Strayer et al., Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 51, 40–47 (2006). https://doi.org/10.1016/j.seppur.2005.12.020
F. David, V. Vokhminz, G. Ionova, Water characteristics depend on the ionic environment. Thermodynamics and modelisation of the aquo ions. J. Mol. Liq. 90, 45–62 (2001). https://doi.org/10.1016/S0167-7322(01)00106-4
M. Śmiechowski, J. Stangret, ATR FT-IR H2O spectra of acidic aqueous solutions. Insights about proton hydration. J. Mol. Struct. 878, 104–115 (2008). https://doi.org/10.1016/j.molstruc.2007.08.001
Y. Chen, Y.-H. Zhang, L.-J. Zhao, ATR-FTIR spectroscopic studies on aqueous LiClO4, NaClO4, and Mg(ClO4)2 solutions. Phys. Chem. Chem. Phys. 6, 537–542 (2004). https://doi.org/10.1039/b311768e
Z. Dou, L. Wang, J. Hu, W. Fang, C. Sun et al., Hydrogen bonding effect on Raman modes of formic acid-water binary solutions. J. Mol. Liq. 313, 113595 (2020). https://doi.org/10.1016/j.molliq.2020.113595
S.L. Simon, Temperature-modulated differential scanning calorimetry: theory and application. Thermochim. Acta 374, 55–71 (2001). https://doi.org/10.1016/S0040-6031(01)00493-2
F. Wan, L. Zhang, X. Dai, X. Wang, Z. Niu et al., Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 9, 1656 (2018). https://doi.org/10.1038/s41467-018-04060-8
M. Zhu, X. Wang, H. Tang, J. Wang, Q. Hao et al., Antifreezing hydrogel with high zinc reversibility for flexible and durable aqueous batteries by cooperative hydrated cations. Adv. Funct. Mater. 30, 1907218 (2019). https://doi.org/10.1002/adfm.201907218
P. Wang, X. Xie, Z. Xing, X. Chen, G. Fang et al., Mechanistic insights of Mg2+-electrolyte additive for high-energy and long-life zinc-ion hybrid capacitors. Adv. Energy Mater. 11, 2102258 (2021). https://doi.org/10.1002/aenm.202101158
T. Sun, C. Liu, X. Xu, Q. Nian, S. Zheng et al., Insights into the hydronium-ion storage of alloxazine in mild electrolyte. J. Mater. Chem. A 8, 21983–21987 (2020). https://doi.org/10.1039/d0ta09316e
Z. Guo, J. Huang, X. Dong, Y. Xia, L. Yan et al., An organic/inorganic electrode-based hydronium-ion battery. Nat. Commun. 11, 959 (2020). https://doi.org/10.1038/s41467-020-14748-5
T. Sun, C. Liu, J. Wang, Q. Nian, Y. Feng et al., A phenazine anode for high-performance aqueous rechargeable batteries in a wide temperature range. Nano Res. 13, 676–683 (2020). https://doi.org/10.1007/s12274-020-2674-3
J. Hong, M. Lee, B. Lee, D.-H. Seo, C.B. Park et al., Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 5, 5335 (2014). https://doi.org/10.1038/ncomms6335