Electrochemical Impedance Analysis of Biofunctionalized Conducting Polymer-Modified Graphene-CNTs Nanocomposite for Protein Detection
Corresponding Author: Rajesh Rajesh
Nano-Micro Letters,
Vol. 9 No. 1 (2017), Article Number: 7
Abstract
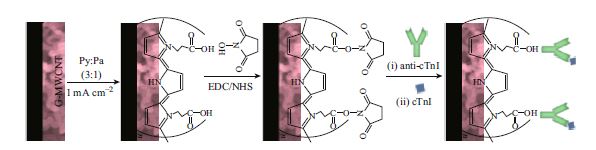
We report an electrodeposited poly(pyrrole-co-pyrrolepropylic acid) copolymer modified electroactive graphene-carbon nanotubes composite deposited on a glassy carbon electrode to detect the protein antigen (cTnI). The copolymer provides pendant carboxyl groups for the site-specific covalent immobilization of protein antibody, anti-troponin I. The hybrid nanocomposite was used as a transducer for biointerfacial impedance sensing for cTnI detection. The results show that the hybrid exhibits a pseudo capacitive behaviour with a maximum phase angle of 49° near 1 Hz, which is due to the inhomogeneous and porous structure of the hybrid composition. The constant phase element of copolymer is 0.61 (n = 0.61), whereas, it is 0.88 (n = 0.88) for the hybrid composites, indicating a comparatively homogeneous microstructure after biomolecular functionalization. The transducer shows a linear change in charge transfer characteristic (R et) on cTnI immunoreaction for spiked human serum in the concentration range of 1.0 pg mL−1–10.0 ng mL−1. The sensitivity of the transducer is 167.8 ± 14.2 Ω cm2 per decade, and it also exhibits high specificity and good reproducibility.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- J. Gong, X. Miao, T. Zhou, L. Zhang, An enzymeless organophosphate pesticide sensor using Au-nanoparticle decorated graphene hybrid nanosheet as solid-phase extraction. Talanta 85(3), 1344–1349 (2011). doi:10.1016/j.talanta.2011.06.016
- J. Lu, I. Do, L.T. Drzal, R.M. Worden, I. Lee, Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano 2(9), 1825–1832 (2008). doi:10.1021/nn800244k
- X. Sun, L. Qiao, X. Wang, A novel immunosensor based on Au nanoparticles and polyaniline/multiwall carbon nanotubes/chitosan nanocomposite film functionalized interface. Nano-Micro Lett. 5(3), 191–201 (2013). doi:10.1007/BF03353750
- D.H. Kim, J.A. Wiler, D.J. Anderson, D.R. Kipke, D.C. Martin, Conducting polymers on hydrogel-coated neural electrode provides sensitive neural recordings in auditory cortex. Acta Biomater. 6(1), 57–62 (2010). doi:10.1016/j.actbio.2009.07.034
- S.M. Richardsons, J.L. Hendricks, B. Foster, Polymerization of the conducting polymer poly (3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials 28(8), 1539–1552 (2007). doi:10.1016/j.biomaterials.2006.11.026
- A. Ramanavicius, A. Ramanaviciene, A. Malinauskas, Electro-chemical sensors based on conducting polymer-polypyrrole. Electrochim. Acta 51, 6025–6037 (2006). doi:10.1016/j.electacta.2005.11.052
- Y.P. Hsiao, Y.W. Su, J.R. Cheng, S.H. Cheng, Electrochemical determination of cysteine based on conducting polymers/gold nanoparticles hybrid nanocomposites. Electrochim. Acta 56(20), 6887–6895 (2011). doi:10.1016/j.electacta.2011.06.031
- J.W. Lee, F. Serna, J. Nickels, C.E. Schimdt, Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules 7(6), 1692–1695 (2006). doi:10.1021/bm060220q
- L.F.Q.P. Marchesi, F.R. Simoes, L.A. Pocrifka, E.C. Pereira, Investigation of polypyrrole degradation using electrochemical impedance spectroscopy. J. Phys. Chem. B 115(31), 9570–9575 (2011). doi:10.1021/jp2041263
- W. Chen, Z. Lu, C.M. Li, Sensitive human interleukin 5 impedimetricimmunosensor based on polypyrrole-pyrrolepropylic acid-gold nanocomposite. Anal. Chem. 80(22), 8485–8492 (2008). doi:10.1021/ac8012225
- D. Wang, W. Hu, Y. Xiong, Y. Xu, C.M. Li, Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetricimmunosensor to ultra-sensitively detect small molecular aflatoxin B1. Biosens. Bioelectron. 63, 185–189 (2015). doi:10.1016/j.bios.2014.06.070
- K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Electric field effect in atomically thin carbon films. Science 306(5696), 666–669 (2004). doi:10.1126/science.1102896
- A. Bonnani, A.H. Loo, M. Pumera, Graphene for impedimetric biosensing. Anal. Chem. 37(37), 12–21 (2012). doi:10.1016/j.trac.2012.02.011
- Y.M. Lin, C. Dimitrakopoulos, K.A. Jenkins, D.B. Farmer, H.Y. Chiu, A. Grill et al., 100-GHz transistors from wafer-scale epitaxial graphene. Science 327(5966), 662 (2010). doi:10.1126/science.1184289
- N. Zhang, M.Q. Yang, S. Liu, Y. Sun, Y.J. Xu, Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 115(18), 10307–10377 (2015). doi:10.1021/acs.chemrev.5b00267
- N. Zhang, Y.J. Xu, The endeavour to advance graphene-semiconductor composite-based photocatalysis. Cryst. Eng. Commun. 18(1), 24–37 (2016). doi:10.1039/C5CE01712B
- N. Zhang, Y. Zhang, Y.J. Xu, Recent progress on graphene-based photocatalysts: current status and future perspectives. Nanoscale 4(19), 5792–5813 (2012). doi:10.1039/c2nr31480k
- S. Paulson, A. Helser, M.B. Nardelli, R.M. Taylor, M. Falvo, R. Superfine, S. Washburn, Tunable resistance of a carbon nanotube-graphite interface. Science 290(5497), 1742–1744 (2000). doi:10.1126/science.290.5497.1742
- F.D. Novaes, R. Rurali, P. Ordejon, Electronic transport between graphene layers covalently connected by carbon nanotubes. ACS Nano 4(12), 7596–7602 (2010). doi:10.1021/nn102206n
- Y.S. Kim, K. Kumar, F.T. Fisher, E. Yang, Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 23(1), 015301 (2012). doi:10.1088/0957-4484/23/1/015301
- K.Y. Hwa, B. Subramani, Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 62(22), 127–133 (2014). doi:10.1016/j.bios.2014.06.023
- B. Kaur, T. Pandiyan, B. Satpati, R. Srivastava, Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloid Surf. B 111(6), 97–106 (2013). doi:10.1016/j.colsurfb.2013.05.023
- P. Ammann, M. Pfisterer, T. Fehr, H. Rickli, Raised cardiac troponins; causes extend beyond acute coronary syndromes. Br. Med. J. 328(7447), 1028–1029 (2004). doi:10.1136/bmj.328.7447.1028
- S. Ko, B. Kim, S.S. Jo, S.Y. Oh, J.K. Park, Electrochemical detection of cardiac troponin I using a microchip with the surface-functionalized poly (dimethylsiloxane) channel. Biosens. Bioelectron. 23(1), 51–59 (2007). doi:10.1016/j.bios.2007.03.013
- A. Qureshi, Y. Gurbuz, J.H. Niazi, Biosensors for cardiac biomarkers detection: a review. Sens. Actuat. B 171–172, 62–76 (2012). doi:10.1016/j.snb.2012.05.077
- R.K. Rajesh, A. Paul, Mulchandani, Platinum nanoflowers decorated three-dimensional graphene-carbon nanotubes hybrid with enhanced electrocatalytic activity. J. Power Sources 223(1), 23–29 (2013). doi:10.1016/j.jpowsour.2012.08.088
- Y. Li, P. Wang, L. Wang, X. Lin, Over oxidized polypyrrole film directed single-walled carbon nanotubes immobilization on glassy carbon electrode and its sensing applications. Biosens. Bioelectron. 22(12), 3120–3125 (2007). doi:10.1016/j.bios.2007.02.001
- B. Rajib, M. Larif, G. Mouhssine, A. Elmidaoui, M.E. Touhami, A. Chaouch, Valorization of polyphenols extracted from olive mill wastewater as ecological corrosion inhibitor on carbon steel in acid medium. Der Pharm. Chem. 8(2), 145–153 (2016)
- B. Derkus, M. Ozkan, K.C. Emregul, E. Emregul, Single frequency analysis for clinical immunosensor design. RSC Adv. 6(1), 281–289 (2016). doi:10.1039/C5RA23783A
- M. Bart, E.C.A. Stigter, H.R. Stapert, G.J. Jong, W.P. Bennekom, On the response of a labl-free interferon –γ immunosensor utilizing electrochemical impedance spectroscopy. Biosens. Bioelectron. 21(1), 49–59 (2005). doi:10.1016/j.bios.2004.10.009
- A. Periyakaruppan, R.P. Gandhiraman, M. Meyyappan, J.E. Koehne, Label-free detection of cardiac troponin-I using carbon nanofiber based nanoelectrode array. Anal. Chem. 85(8), 3858–3863 (2013). doi:10.1021/ac302801z
- Z.R. Guo, C.R. Gu, X. Fan, Z.P. Bian, H.F. Wu, D. Yang, N. Gu, J.N. Zhang, Fabrication of anti-human cardiac troponin I immunogold nanorods for sensing acute myocardial damage. Nanoscale Res. Lett. 4(12), 1428–1433 (2009). doi:10.1007/s11671-009-9415-6
- A.J.S. Ahammad, Y.H. Choi, K. Koh, J.H. Kim, J.J. Lee, M. Lee, Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochim. Sci. 6(6), 1906–1916 (2011)
- A.A. Shumkov, E.V. Suprun, S.Z. Shatinina, A.V. Lisitsa, V.V. Shumyantseva, A.I. Archakov, Gold and silver nanoparticles for electrochemical detection of cardiac troponin I based on stripping voltammetry. J. Bionanosci. 3(2), 216–222 (2013). doi:10.1007/s12668-013-0090-9
- W.Y. Wu, Z.P. Bian, W. Wang, W. Wang, J.J. Zhu, PDMS gold nanoparticle composite film-based silver enhanced colorimetric detection of cardiac troponin I. Sens. Actuat. B 147(1), 298–303 (2010). doi:10.1016/j.snb.2010.03.027
- Y. Ryu, Z. Jin, M.S. Kang, H.S. Kim, Increase in the detection sensitivity of a lateral flow assay for a cardiac marker by oriented immobilization of antibody. Biochip J. 5, 193–198 (2011). doi:10.1007/s13206-011-5301-2
References
J. Gong, X. Miao, T. Zhou, L. Zhang, An enzymeless organophosphate pesticide sensor using Au-nanoparticle decorated graphene hybrid nanosheet as solid-phase extraction. Talanta 85(3), 1344–1349 (2011). doi:10.1016/j.talanta.2011.06.016
J. Lu, I. Do, L.T. Drzal, R.M. Worden, I. Lee, Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano 2(9), 1825–1832 (2008). doi:10.1021/nn800244k
X. Sun, L. Qiao, X. Wang, A novel immunosensor based on Au nanoparticles and polyaniline/multiwall carbon nanotubes/chitosan nanocomposite film functionalized interface. Nano-Micro Lett. 5(3), 191–201 (2013). doi:10.1007/BF03353750
D.H. Kim, J.A. Wiler, D.J. Anderson, D.R. Kipke, D.C. Martin, Conducting polymers on hydrogel-coated neural electrode provides sensitive neural recordings in auditory cortex. Acta Biomater. 6(1), 57–62 (2010). doi:10.1016/j.actbio.2009.07.034
S.M. Richardsons, J.L. Hendricks, B. Foster, Polymerization of the conducting polymer poly (3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials 28(8), 1539–1552 (2007). doi:10.1016/j.biomaterials.2006.11.026
A. Ramanavicius, A. Ramanaviciene, A. Malinauskas, Electro-chemical sensors based on conducting polymer-polypyrrole. Electrochim. Acta 51, 6025–6037 (2006). doi:10.1016/j.electacta.2005.11.052
Y.P. Hsiao, Y.W. Su, J.R. Cheng, S.H. Cheng, Electrochemical determination of cysteine based on conducting polymers/gold nanoparticles hybrid nanocomposites. Electrochim. Acta 56(20), 6887–6895 (2011). doi:10.1016/j.electacta.2011.06.031
J.W. Lee, F. Serna, J. Nickels, C.E. Schimdt, Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules 7(6), 1692–1695 (2006). doi:10.1021/bm060220q
L.F.Q.P. Marchesi, F.R. Simoes, L.A. Pocrifka, E.C. Pereira, Investigation of polypyrrole degradation using electrochemical impedance spectroscopy. J. Phys. Chem. B 115(31), 9570–9575 (2011). doi:10.1021/jp2041263
W. Chen, Z. Lu, C.M. Li, Sensitive human interleukin 5 impedimetricimmunosensor based on polypyrrole-pyrrolepropylic acid-gold nanocomposite. Anal. Chem. 80(22), 8485–8492 (2008). doi:10.1021/ac8012225
D. Wang, W. Hu, Y. Xiong, Y. Xu, C.M. Li, Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetricimmunosensor to ultra-sensitively detect small molecular aflatoxin B1. Biosens. Bioelectron. 63, 185–189 (2015). doi:10.1016/j.bios.2014.06.070
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Electric field effect in atomically thin carbon films. Science 306(5696), 666–669 (2004). doi:10.1126/science.1102896
A. Bonnani, A.H. Loo, M. Pumera, Graphene for impedimetric biosensing. Anal. Chem. 37(37), 12–21 (2012). doi:10.1016/j.trac.2012.02.011
Y.M. Lin, C. Dimitrakopoulos, K.A. Jenkins, D.B. Farmer, H.Y. Chiu, A. Grill et al., 100-GHz transistors from wafer-scale epitaxial graphene. Science 327(5966), 662 (2010). doi:10.1126/science.1184289
N. Zhang, M.Q. Yang, S. Liu, Y. Sun, Y.J. Xu, Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 115(18), 10307–10377 (2015). doi:10.1021/acs.chemrev.5b00267
N. Zhang, Y.J. Xu, The endeavour to advance graphene-semiconductor composite-based photocatalysis. Cryst. Eng. Commun. 18(1), 24–37 (2016). doi:10.1039/C5CE01712B
N. Zhang, Y. Zhang, Y.J. Xu, Recent progress on graphene-based photocatalysts: current status and future perspectives. Nanoscale 4(19), 5792–5813 (2012). doi:10.1039/c2nr31480k
S. Paulson, A. Helser, M.B. Nardelli, R.M. Taylor, M. Falvo, R. Superfine, S. Washburn, Tunable resistance of a carbon nanotube-graphite interface. Science 290(5497), 1742–1744 (2000). doi:10.1126/science.290.5497.1742
F.D. Novaes, R. Rurali, P. Ordejon, Electronic transport between graphene layers covalently connected by carbon nanotubes. ACS Nano 4(12), 7596–7602 (2010). doi:10.1021/nn102206n
Y.S. Kim, K. Kumar, F.T. Fisher, E. Yang, Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 23(1), 015301 (2012). doi:10.1088/0957-4484/23/1/015301
K.Y. Hwa, B. Subramani, Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 62(22), 127–133 (2014). doi:10.1016/j.bios.2014.06.023
B. Kaur, T. Pandiyan, B. Satpati, R. Srivastava, Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloid Surf. B 111(6), 97–106 (2013). doi:10.1016/j.colsurfb.2013.05.023
P. Ammann, M. Pfisterer, T. Fehr, H. Rickli, Raised cardiac troponins; causes extend beyond acute coronary syndromes. Br. Med. J. 328(7447), 1028–1029 (2004). doi:10.1136/bmj.328.7447.1028
S. Ko, B. Kim, S.S. Jo, S.Y. Oh, J.K. Park, Electrochemical detection of cardiac troponin I using a microchip with the surface-functionalized poly (dimethylsiloxane) channel. Biosens. Bioelectron. 23(1), 51–59 (2007). doi:10.1016/j.bios.2007.03.013
A. Qureshi, Y. Gurbuz, J.H. Niazi, Biosensors for cardiac biomarkers detection: a review. Sens. Actuat. B 171–172, 62–76 (2012). doi:10.1016/j.snb.2012.05.077
R.K. Rajesh, A. Paul, Mulchandani, Platinum nanoflowers decorated three-dimensional graphene-carbon nanotubes hybrid with enhanced electrocatalytic activity. J. Power Sources 223(1), 23–29 (2013). doi:10.1016/j.jpowsour.2012.08.088
Y. Li, P. Wang, L. Wang, X. Lin, Over oxidized polypyrrole film directed single-walled carbon nanotubes immobilization on glassy carbon electrode and its sensing applications. Biosens. Bioelectron. 22(12), 3120–3125 (2007). doi:10.1016/j.bios.2007.02.001
B. Rajib, M. Larif, G. Mouhssine, A. Elmidaoui, M.E. Touhami, A. Chaouch, Valorization of polyphenols extracted from olive mill wastewater as ecological corrosion inhibitor on carbon steel in acid medium. Der Pharm. Chem. 8(2), 145–153 (2016)
B. Derkus, M. Ozkan, K.C. Emregul, E. Emregul, Single frequency analysis for clinical immunosensor design. RSC Adv. 6(1), 281–289 (2016). doi:10.1039/C5RA23783A
M. Bart, E.C.A. Stigter, H.R. Stapert, G.J. Jong, W.P. Bennekom, On the response of a labl-free interferon –γ immunosensor utilizing electrochemical impedance spectroscopy. Biosens. Bioelectron. 21(1), 49–59 (2005). doi:10.1016/j.bios.2004.10.009
A. Periyakaruppan, R.P. Gandhiraman, M. Meyyappan, J.E. Koehne, Label-free detection of cardiac troponin-I using carbon nanofiber based nanoelectrode array. Anal. Chem. 85(8), 3858–3863 (2013). doi:10.1021/ac302801z
Z.R. Guo, C.R. Gu, X. Fan, Z.P. Bian, H.F. Wu, D. Yang, N. Gu, J.N. Zhang, Fabrication of anti-human cardiac troponin I immunogold nanorods for sensing acute myocardial damage. Nanoscale Res. Lett. 4(12), 1428–1433 (2009). doi:10.1007/s11671-009-9415-6
A.J.S. Ahammad, Y.H. Choi, K. Koh, J.H. Kim, J.J. Lee, M. Lee, Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochim. Sci. 6(6), 1906–1916 (2011)
A.A. Shumkov, E.V. Suprun, S.Z. Shatinina, A.V. Lisitsa, V.V. Shumyantseva, A.I. Archakov, Gold and silver nanoparticles for electrochemical detection of cardiac troponin I based on stripping voltammetry. J. Bionanosci. 3(2), 216–222 (2013). doi:10.1007/s12668-013-0090-9
W.Y. Wu, Z.P. Bian, W. Wang, W. Wang, J.J. Zhu, PDMS gold nanoparticle composite film-based silver enhanced colorimetric detection of cardiac troponin I. Sens. Actuat. B 147(1), 298–303 (2010). doi:10.1016/j.snb.2010.03.027
Y. Ryu, Z. Jin, M.S. Kang, H.S. Kim, Increase in the detection sensitivity of a lateral flow assay for a cardiac marker by oriented immobilization of antibody. Biochip J. 5, 193–198 (2011). doi:10.1007/s13206-011-5301-2