Identification of Dynamic Active Sites Among Cu Species Derived from MOFs@CuPc for Electrocatalytic Nitrate Reduction Reaction to Ammonia
Corresponding Author: Jun Tao
Nano-Micro Letters,
Vol. 15 (2023), Article Number: 110
Abstract
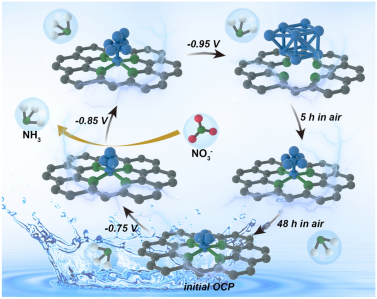
Direct electrochemical nitrate reduction reaction (NITRR) is a promising strategy to alleviate the unbalanced nitrogen cycle while achieving the electrosynthesis of ammonia. However, the restructuration of the high-activity Cu-based electrocatalysts in the NITRR process has hindered the identification of dynamical active sites and in-depth investigation of the catalytic mechanism. Herein, Cu species (single-atom, clusters, and nanoparticles) with tunable loading supported on N-doped TiO2/C are successfully manufactured with MOFs@CuPc precursors via the pre-anchor and post-pyrolysis strategy. Restructuration behavior among Cu species is co-dependent on the Cu loading and reaction potential, as evidenced by the advanced operando X-ray absorption spectroscopy, and there exists an incompletely reversible transformation of the restructured structure to the initial state. Notably, restructured CuN4&Cu4 deliver the high NH3 yield of 88.2 mmol h−1 gcata−1 and FE (~ 94.3%) at − 0.75 V, resulting from the optimal adsorption of NO3− as well as the rapid conversion of *NH2OH to *NH2 intermediates originated from the modulation of charge distribution and d-band center for Cu site. This work not only uncovers CuN4&Cu4 have the promising NITRR but also identifies the dynamic Cu species active sites that play a critical role in the efficient electrocatalytic reduction in nitrate to ammonia.
Highlights:
1 Cu species with tunable loading supported on N-doped TiO2/C were successfully fabricated utilizing MOFs@CuPc precursors via the pre-anchor and post-pyrolysis strategy.
2 Cu species with tunable loading supported on N-doped TiO2/C were successfully fabricated utilizing MOFs@CuPc precursors via the pre-anchor and post-pyrolysis strategy.
3 Restructured CuN4&Cu4 performed the highest NH3 yield (88.2 mmol h−1 gcata−1) and FE (~94.3%) at − 0.75 V due to optimal adsorption of NO3− and rapid conversion of the key intermediates.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- C.X. Guo, J.R. Ran, A. Vasileff, S.Z. Qiao, Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 11(1), 45–56 (2018). https://doi.org/10.1039/C7EE022-20D
- W.H. Guo, K.X. Zhang, Z.B. Liang, R.Q. Zou, Q. Xu, Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design. Chem. Soc. Rev. 48(24), 5658–5716 (2019). https://doi.org/10.1039/C9CS00159J
- W.C. Zhang, B.W. Zhang, Bi-atom electrocatalyst for electrochemical nitrogen reduction reactions. Nano Micro Lett. 13, 106 (2021). https://doi.org/10.1007/s40820-021-00638-y
- Y.P. Pang, C. Su, G.H. Jia, L.Q. Xu, Z.P. Shao, Emerging two-dimensional nanomaterials for electrochemical nitrogen reduction. Chem. Soc. Rev. 50(22), 12744–12787 (2021). https://doi.org/10.1039/D1CS00120E
- A. Biswas, S. Kapse, R. Thapa, R.S. Dey, Oxygen functionalization-induced charging effect on boron active sites for high-yield electrocatalytic NH3 production. Nano Micro Lett. 14, 214 (2022). https://doi.org/10.1007/s40820-022-00966-7
- X.W. Wang, D. Wu, S.Y. Liu, J.J. Zhang, X.Z. Fu et al., Folic acid self-assembly enabling manganese single-atom electrocatalyst for selective nitrogen reduction to ammonia. Nano Micro Lett. 13, 125 (2021). https://doi.org/10.1007/s40820-021-00651-1
- Y.T. Wang, C.H. Wang, M.Y. Li, Y.F. Yu, B. Zhang, Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 50(12), 6720–6733 (2021). https://doi.org/10.1039/D1CS00116G
- H. Xu, Y.Y. Ma, J. Chen, W.X. Zhang, J.P. Yang, Electrocatalytic reduction of nitrate–a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 51(7), 2710–2758 (2022). https://doi.org/10.1039/D1CS00857A
- R. Zhang, Y. Guo, S.C. Zhang, D. Chen, Y.W. Zhao et al., Efficient ammonia electrosynthesis and energy conversion through a Zn-nitrate battery by iron doping engineered nickel phosphide catalyst. Adv. Energy Mater. 12(13), 2103872 (2022). https://doi.org/10.1002/aenm.202103872
- G.F. Chen, Y.F. Yuan, H.F. Jiang, S.Y. Ren, L.X. Ding et al., Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5(8), 605–613 (2020). https://doi.org/10.1038/s41560-020-0654-1
- D.M. Zhao, Y.Q. Wang, C.L. Dong, F.Q. Meng, Y.C. Huang et al., Electron-deficient Zn-N6 configuration enabling polymeric carbon nitride for visible-light photocatalytic overall water splitting. Nano Micro Lett. 14, 223 (2022). https://doi.org/10.1007/s40820-022-00962-x
- L.L. Han, H. Cheng, W. Liu, H.Q. Li, P.F. Ou et al., A single-atom library for guided monometallic and concentration-complex multimetallic designs. Nat. Mater. 21(6), 681–688 (2022). https://doi.org/10.1038/s41563-022-01252-y
- X. Wu, H.B. Zhang, S.W. Zuo, J.C. Dong, Y. Li et al., Engineering the coordination sphere of isolated active sites to explore the intrinsic activity in single-atom catalysts. Nano Micro Lett. 13, 136 (2021). https://doi.org/10.1007/s40820-021-00668-6
- X.B. Zheng, P. Li, S.X. Dou, W.P. Sun, H.G. Pan et al., Non-carbon-supported single-atom site catalysts for electrocatalysis. Energy Environ. Sci. 14(5), 2809–2858 (2021). https://doi.org/10.1039/D1EE00248A
- W.X. Chen, J.J. Pei, C.T. He, J.W. Wan, H.L. Ren et al., Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution. Adv. Mater. 30(30), 180039 (2018). https://doi.org/10.1002/adma.201800396
- B.L. Yan, D.P. Liu, X.L. Feng, M.Z. Shao, Y. Zhang, Ru species supported on MOF-derived N-doped TiO2/C hybrids as efficient electrocatalytic/photocatalytic hydrogen evolution reaction catalysts. Adv. Funct. Mater. 30(31), 2003 (2020). https://doi.org/10.1002/adfm.202003007
- X.Y. Xie, L.S. Peng, H.Z. Yang, G.I.N. Waterhouse, L. Shang et al., MIL-101-derived mesoporous carbon supporting highly exposed Fe single-atom sites as efficient oxygen reduction reaction catalysts. Adv. Mater. 33(23), 21010 (2021). https://doi.org/10.1002/adma.202101038
- J. Choi, P. Wagner, S. Gambhir, R. Jalili, D.R. MacFarlane et al., Steric modification of a cobalt phthalocyanine/graphene catalyst to give enhanced and stable electrochemical CO2 reduction to CO. ACS Energy Lett. 4(3), 666–672 (2019). https://doi.org/10.1021/acsenergylett.8b02355
- C. Xia, Y.R. Qiu, Y. Xia, P. Zhu, G. King et al., General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13(9), 887–894 (2021). https://doi.org/10.1038/s41557-021-00734-x
- W.J. Zhai, S.H. Huang, C.B. Lu, X.N. Tang, L.B. Li et al., Simultaneously integrate iron single atom and nanocluster triggered tandem effect for boosting oxygen electroreduction. Small 18(15), 2107225 (2022). https://doi.org/10.1002/smll.202107225
- Z.M. Ma, S.Q. Liu, N.F. Tang, T. Song, K. Motokura et al., Coexistence of Fe nanoclusters boosting Fe single atoms to generate singlet oxygen for efficient aerobic oxidation of primary amines to imines. ACS Catal. 12(9), 5595–5604 (2022). https://doi.org/10.1021/acscatal.1c04467
- G.Y. Xing, M.M. Tong, P. Yu, L. Wang, G.Y. Zhang et al., Restructuration of highly dense Cu−N4 active sites in electrocatalytic oxygen reduction characterized by operando synchrotron radiation. Angew. Chem. Int. Ed. 61(40), e202211 (2022). https://doi.org/10.1002/anie.202211098
- H.P. Xu, D. Rebollar, H.Y. He, L.N. Chong, Y.Z. Liu et al., Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5(8), 623–632 (2020). https://doi.org/10.1038/s41560-020-0666-x
- J. Yang, H.F. Qi, A.Q. Li, X.Y. Liu, X.F. Yang et al., Potential-driven restructuring of Cu single atoms to nanops for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 144(27), 12062–12071 (2022). https://doi.org/10.1021/jacs.2c02262
- Y.T. Xu, M.Y. Xie, H.Q. Zhong, Y. Cao, In situ clustering of single-atom copper precatalysts in a metal-organic framework for efficient electrocatalytic nitrate-to-ammonia reduction. ACS Catal. 12(14), 8698–8706 (2022). https://doi.org/10.1021/acscatal.2c02033
- X.Y. Ji, Y.Y. Wang, Y. Li, K. Sun, M. Yu et al., Enhancing photocatalytic hydrogen peroxide production of Ti-based metal–organic frameworks: the leading role of facet engineering. Nano Res. 15(7), 6045–6053 (2022). https://doi.org/10.1007/s12274-022-4301-y
- J. Janczak, Y.M. Idemori, Synthesis, crystal structure and characterisation of aquamagnesium phthalocyanine—MgPc(H2O). The origin of an intense near-IR absorption of magnesium phthalocyanine known as ‘X-phase.’ Polyhedron 22(9), 1167–1181 (2003). https://doi.org/10.1016/S0277-5387(03)00110-4
- J. Gu, C.S. Hsu, L.C. Bai, H.M. Chen, X.L. Hu, Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364(6445), 1091–1094 (2019). https://doi.org/10.1126/science.aaw7515
- H.D. Li, Y. Pan, Z.C. Wang, Y.D. Yu, J. Xiong et al., Coordination engineering of cobalt phthalocyanine by functionalized carbon nanotube for efficient and highly stable carbon dioxide reduction at high current density. Nano Res. 15(4), 3056–3064 (2022). https://doi.org/10.1007/s12274-021-3962-2
- Q.W. Chang, Y.M. Liu, J.H. Lee, D. Ologunagba, S. Hwang et al., Metal-coordinated phthalocyanines as platform molecules for understanding isolated metal sites in the electrochemical reduction of CO2. J. Am. Chem. Soc. 144(35), 16131–16138 (2022). https://doi.org/10.1021/jacs.2c06953
- M.Y. Li, T.H. Wang, W.X. Zhao, S.Y. Wang, Y.Q. Zou, A pair-electrosynthesis for formate at ultra-low voltage via coupling of CO2 reduction and formaldehyde oxidation. Nano Micro Lett. 14, 211 (2022). https://doi.org/10.1007/s40820-022-00953-y
- W. Zhou, L. Fu, L. Zhao, X.J. Xu, W.Y. Li et al., Novel core–sheath Cu/Cu2O-ZnO-Fe3O4 nanocomposites with high-efficiency chlorine-resistant bacteria sterilization and trichloroacetic acid degradation performance. ACS Appl. Mater. Interfaces 13(9), 10878–10890 (2021). https://doi.org/10.1021/acsami.0c21336
- X. Zhang, C.H. Wang, Y.M. Guo, B. Zhang, Y.T. Wang et al., Cu clusters/TiO2–x with abundant oxygen vacancies for enhanced electrocatalytic nitrate reduction to ammonia. J. Mater. Chem. A 10(12), 6448–6453 (2022). https://doi.org/10.1039/D2TA00661H
- H. Su, W.L. Zhou, H. Zhang, W. Zhou, X. Zhao et al., Dynamic evolution of solid−liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 142(28), 12306–12313 (2020). https://doi.org/10.1021/jacs.0c04231
- D. Karapinar, N.T. Huan, N.R. Sahraie, J.K. Li, D. Wakerley et al., Electroreduction of CO2 on single-site copper-nitrogen-doped carbon material: selective formation of ethanol and reversible restructuration of the metal sites. Angew. Chem. Int. Ed. 58(42), 15098 (2019). https://doi.org/10.1002/anie.201907994
- X.Z. Su, Z.L. Jiang, J. Zhou, H.J. Liu, D.N. Zhou et al., Complementary Operando Spectroscopy identification of in-situ generated metastable charge-asymmetry Cu2-CuN3 clusters for CO2 reduction to ethanol. Nat. Commun. 13, 1322 (2022). https://doi.org/10.1038/s41467-022-29035-8
- J. Yang, W.G. Liu, M.Q. Xu, X.Y. Liu, H.F. Qi et al., Dynamic behavior of single-atom catalysts in electrocatalysis: identification of Cu-N3 as an active site for the oxygen reduction reaction. J. Am. Chem. Soc. 143(36), 14530–14539 (2021). https://doi.org/10.1021/jacs.1c03788
- X. Ao, W. Zhang, Z.S. Li, J.G. Li, L. Soule et al., Markedly enhanced oxygen reduction activity of single-atom Fe catalysts via integration with Fe nanoclusters. ACS Nano 13(10), 11853–11862 (2019). https://doi.org/10.1021/acsnano.9b05913
- X. Wan, Q.T. Liu, J.Y. Liu, S.Y. Liu, X.F. Liu et al., Iron atom–cluster interactions increase activity and improve durability in Fe–N–C fuel cells. Nat. Commun. 13, 2963 (2022). https://doi.org/10.1038/s41467-022-30702-z
- X.H. Liu, L.R. Zheng, C.X. Han, H.X. Zong, G. Yang et al., Identifying the activity origin of a cobalt single-atom catalyst for hydrogen evolution using supervised learning. Adv. Funct. Mater. 31(18), 2100547 (2021). https://doi.org/10.1002/adfm.202100547
- Q. Hu, Y.J. Qin, X.D. Wang, Z.Y. Wang, X.W. Huang et al., Reaction intermediate-mediated electrocatalyst synthesis favors specified facet and defect exposure for efficient nitrate–ammonia conversion. Energy Environ. Sci. 14(9), 4989–4997 (2021). https://doi.org/10.1039/D1EE01731D
- A. Kumar, J. Lee, M.G. Kim, B. Debnath, X.H. Liu et al., Efficient nitrate conversion to ammonia on f-block single-atom/metal oxide heterostructure via local electron-deficiency modulation. ACS Nano 16(9), 15297–15309 (2022). https://doi.org/10.1021/acsnano.2c06747
- Y.T. Wang, H.J. Li, W. Zhou, X. Zhang, B. Zhang et al., Structurally disordered RuO2 nanosheets with rich oxygen vacancies for enhanced nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. 61(19), e202202604 (2022). https://doi.org/10.1002/anie.202202604
- J. Li, G.M. Zhan, J.H. Yang, F.J. Quan, C.L. Mao et al., Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142(15), 7036–7046 (2020). https://doi.org/10.1021/jacs.0c00418
- H.B. Yin, Z. Chen, S.C. Xiong, J.J. Chen, C.Z. Wang et al., Alloying effect-induced electron polarization drives nitrate electroreduction to ammonia. Chem. Catal. 1(5), 1088–1103 (2021). https://doi.org/10.1016/j.checat.2021.08.014
References
C.X. Guo, J.R. Ran, A. Vasileff, S.Z. Qiao, Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 11(1), 45–56 (2018). https://doi.org/10.1039/C7EE022-20D
W.H. Guo, K.X. Zhang, Z.B. Liang, R.Q. Zou, Q. Xu, Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design. Chem. Soc. Rev. 48(24), 5658–5716 (2019). https://doi.org/10.1039/C9CS00159J
W.C. Zhang, B.W. Zhang, Bi-atom electrocatalyst for electrochemical nitrogen reduction reactions. Nano Micro Lett. 13, 106 (2021). https://doi.org/10.1007/s40820-021-00638-y
Y.P. Pang, C. Su, G.H. Jia, L.Q. Xu, Z.P. Shao, Emerging two-dimensional nanomaterials for electrochemical nitrogen reduction. Chem. Soc. Rev. 50(22), 12744–12787 (2021). https://doi.org/10.1039/D1CS00120E
A. Biswas, S. Kapse, R. Thapa, R.S. Dey, Oxygen functionalization-induced charging effect on boron active sites for high-yield electrocatalytic NH3 production. Nano Micro Lett. 14, 214 (2022). https://doi.org/10.1007/s40820-022-00966-7
X.W. Wang, D. Wu, S.Y. Liu, J.J. Zhang, X.Z. Fu et al., Folic acid self-assembly enabling manganese single-atom electrocatalyst for selective nitrogen reduction to ammonia. Nano Micro Lett. 13, 125 (2021). https://doi.org/10.1007/s40820-021-00651-1
Y.T. Wang, C.H. Wang, M.Y. Li, Y.F. Yu, B. Zhang, Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 50(12), 6720–6733 (2021). https://doi.org/10.1039/D1CS00116G
H. Xu, Y.Y. Ma, J. Chen, W.X. Zhang, J.P. Yang, Electrocatalytic reduction of nitrate–a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 51(7), 2710–2758 (2022). https://doi.org/10.1039/D1CS00857A
R. Zhang, Y. Guo, S.C. Zhang, D. Chen, Y.W. Zhao et al., Efficient ammonia electrosynthesis and energy conversion through a Zn-nitrate battery by iron doping engineered nickel phosphide catalyst. Adv. Energy Mater. 12(13), 2103872 (2022). https://doi.org/10.1002/aenm.202103872
G.F. Chen, Y.F. Yuan, H.F. Jiang, S.Y. Ren, L.X. Ding et al., Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5(8), 605–613 (2020). https://doi.org/10.1038/s41560-020-0654-1
D.M. Zhao, Y.Q. Wang, C.L. Dong, F.Q. Meng, Y.C. Huang et al., Electron-deficient Zn-N6 configuration enabling polymeric carbon nitride for visible-light photocatalytic overall water splitting. Nano Micro Lett. 14, 223 (2022). https://doi.org/10.1007/s40820-022-00962-x
L.L. Han, H. Cheng, W. Liu, H.Q. Li, P.F. Ou et al., A single-atom library for guided monometallic and concentration-complex multimetallic designs. Nat. Mater. 21(6), 681–688 (2022). https://doi.org/10.1038/s41563-022-01252-y
X. Wu, H.B. Zhang, S.W. Zuo, J.C. Dong, Y. Li et al., Engineering the coordination sphere of isolated active sites to explore the intrinsic activity in single-atom catalysts. Nano Micro Lett. 13, 136 (2021). https://doi.org/10.1007/s40820-021-00668-6
X.B. Zheng, P. Li, S.X. Dou, W.P. Sun, H.G. Pan et al., Non-carbon-supported single-atom site catalysts for electrocatalysis. Energy Environ. Sci. 14(5), 2809–2858 (2021). https://doi.org/10.1039/D1EE00248A
W.X. Chen, J.J. Pei, C.T. He, J.W. Wan, H.L. Ren et al., Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution. Adv. Mater. 30(30), 180039 (2018). https://doi.org/10.1002/adma.201800396
B.L. Yan, D.P. Liu, X.L. Feng, M.Z. Shao, Y. Zhang, Ru species supported on MOF-derived N-doped TiO2/C hybrids as efficient electrocatalytic/photocatalytic hydrogen evolution reaction catalysts. Adv. Funct. Mater. 30(31), 2003 (2020). https://doi.org/10.1002/adfm.202003007
X.Y. Xie, L.S. Peng, H.Z. Yang, G.I.N. Waterhouse, L. Shang et al., MIL-101-derived mesoporous carbon supporting highly exposed Fe single-atom sites as efficient oxygen reduction reaction catalysts. Adv. Mater. 33(23), 21010 (2021). https://doi.org/10.1002/adma.202101038
J. Choi, P. Wagner, S. Gambhir, R. Jalili, D.R. MacFarlane et al., Steric modification of a cobalt phthalocyanine/graphene catalyst to give enhanced and stable electrochemical CO2 reduction to CO. ACS Energy Lett. 4(3), 666–672 (2019). https://doi.org/10.1021/acsenergylett.8b02355
C. Xia, Y.R. Qiu, Y. Xia, P. Zhu, G. King et al., General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13(9), 887–894 (2021). https://doi.org/10.1038/s41557-021-00734-x
W.J. Zhai, S.H. Huang, C.B. Lu, X.N. Tang, L.B. Li et al., Simultaneously integrate iron single atom and nanocluster triggered tandem effect for boosting oxygen electroreduction. Small 18(15), 2107225 (2022). https://doi.org/10.1002/smll.202107225
Z.M. Ma, S.Q. Liu, N.F. Tang, T. Song, K. Motokura et al., Coexistence of Fe nanoclusters boosting Fe single atoms to generate singlet oxygen for efficient aerobic oxidation of primary amines to imines. ACS Catal. 12(9), 5595–5604 (2022). https://doi.org/10.1021/acscatal.1c04467
G.Y. Xing, M.M. Tong, P. Yu, L. Wang, G.Y. Zhang et al., Restructuration of highly dense Cu−N4 active sites in electrocatalytic oxygen reduction characterized by operando synchrotron radiation. Angew. Chem. Int. Ed. 61(40), e202211 (2022). https://doi.org/10.1002/anie.202211098
H.P. Xu, D. Rebollar, H.Y. He, L.N. Chong, Y.Z. Liu et al., Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5(8), 623–632 (2020). https://doi.org/10.1038/s41560-020-0666-x
J. Yang, H.F. Qi, A.Q. Li, X.Y. Liu, X.F. Yang et al., Potential-driven restructuring of Cu single atoms to nanops for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 144(27), 12062–12071 (2022). https://doi.org/10.1021/jacs.2c02262
Y.T. Xu, M.Y. Xie, H.Q. Zhong, Y. Cao, In situ clustering of single-atom copper precatalysts in a metal-organic framework for efficient electrocatalytic nitrate-to-ammonia reduction. ACS Catal. 12(14), 8698–8706 (2022). https://doi.org/10.1021/acscatal.2c02033
X.Y. Ji, Y.Y. Wang, Y. Li, K. Sun, M. Yu et al., Enhancing photocatalytic hydrogen peroxide production of Ti-based metal–organic frameworks: the leading role of facet engineering. Nano Res. 15(7), 6045–6053 (2022). https://doi.org/10.1007/s12274-022-4301-y
J. Janczak, Y.M. Idemori, Synthesis, crystal structure and characterisation of aquamagnesium phthalocyanine—MgPc(H2O). The origin of an intense near-IR absorption of magnesium phthalocyanine known as ‘X-phase.’ Polyhedron 22(9), 1167–1181 (2003). https://doi.org/10.1016/S0277-5387(03)00110-4
J. Gu, C.S. Hsu, L.C. Bai, H.M. Chen, X.L. Hu, Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364(6445), 1091–1094 (2019). https://doi.org/10.1126/science.aaw7515
H.D. Li, Y. Pan, Z.C. Wang, Y.D. Yu, J. Xiong et al., Coordination engineering of cobalt phthalocyanine by functionalized carbon nanotube for efficient and highly stable carbon dioxide reduction at high current density. Nano Res. 15(4), 3056–3064 (2022). https://doi.org/10.1007/s12274-021-3962-2
Q.W. Chang, Y.M. Liu, J.H. Lee, D. Ologunagba, S. Hwang et al., Metal-coordinated phthalocyanines as platform molecules for understanding isolated metal sites in the electrochemical reduction of CO2. J. Am. Chem. Soc. 144(35), 16131–16138 (2022). https://doi.org/10.1021/jacs.2c06953
M.Y. Li, T.H. Wang, W.X. Zhao, S.Y. Wang, Y.Q. Zou, A pair-electrosynthesis for formate at ultra-low voltage via coupling of CO2 reduction and formaldehyde oxidation. Nano Micro Lett. 14, 211 (2022). https://doi.org/10.1007/s40820-022-00953-y
W. Zhou, L. Fu, L. Zhao, X.J. Xu, W.Y. Li et al., Novel core–sheath Cu/Cu2O-ZnO-Fe3O4 nanocomposites with high-efficiency chlorine-resistant bacteria sterilization and trichloroacetic acid degradation performance. ACS Appl. Mater. Interfaces 13(9), 10878–10890 (2021). https://doi.org/10.1021/acsami.0c21336
X. Zhang, C.H. Wang, Y.M. Guo, B. Zhang, Y.T. Wang et al., Cu clusters/TiO2–x with abundant oxygen vacancies for enhanced electrocatalytic nitrate reduction to ammonia. J. Mater. Chem. A 10(12), 6448–6453 (2022). https://doi.org/10.1039/D2TA00661H
H. Su, W.L. Zhou, H. Zhang, W. Zhou, X. Zhao et al., Dynamic evolution of solid−liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 142(28), 12306–12313 (2020). https://doi.org/10.1021/jacs.0c04231
D. Karapinar, N.T. Huan, N.R. Sahraie, J.K. Li, D. Wakerley et al., Electroreduction of CO2 on single-site copper-nitrogen-doped carbon material: selective formation of ethanol and reversible restructuration of the metal sites. Angew. Chem. Int. Ed. 58(42), 15098 (2019). https://doi.org/10.1002/anie.201907994
X.Z. Su, Z.L. Jiang, J. Zhou, H.J. Liu, D.N. Zhou et al., Complementary Operando Spectroscopy identification of in-situ generated metastable charge-asymmetry Cu2-CuN3 clusters for CO2 reduction to ethanol. Nat. Commun. 13, 1322 (2022). https://doi.org/10.1038/s41467-022-29035-8
J. Yang, W.G. Liu, M.Q. Xu, X.Y. Liu, H.F. Qi et al., Dynamic behavior of single-atom catalysts in electrocatalysis: identification of Cu-N3 as an active site for the oxygen reduction reaction. J. Am. Chem. Soc. 143(36), 14530–14539 (2021). https://doi.org/10.1021/jacs.1c03788
X. Ao, W. Zhang, Z.S. Li, J.G. Li, L. Soule et al., Markedly enhanced oxygen reduction activity of single-atom Fe catalysts via integration with Fe nanoclusters. ACS Nano 13(10), 11853–11862 (2019). https://doi.org/10.1021/acsnano.9b05913
X. Wan, Q.T. Liu, J.Y. Liu, S.Y. Liu, X.F. Liu et al., Iron atom–cluster interactions increase activity and improve durability in Fe–N–C fuel cells. Nat. Commun. 13, 2963 (2022). https://doi.org/10.1038/s41467-022-30702-z
X.H. Liu, L.R. Zheng, C.X. Han, H.X. Zong, G. Yang et al., Identifying the activity origin of a cobalt single-atom catalyst for hydrogen evolution using supervised learning. Adv. Funct. Mater. 31(18), 2100547 (2021). https://doi.org/10.1002/adfm.202100547
Q. Hu, Y.J. Qin, X.D. Wang, Z.Y. Wang, X.W. Huang et al., Reaction intermediate-mediated electrocatalyst synthesis favors specified facet and defect exposure for efficient nitrate–ammonia conversion. Energy Environ. Sci. 14(9), 4989–4997 (2021). https://doi.org/10.1039/D1EE01731D
A. Kumar, J. Lee, M.G. Kim, B. Debnath, X.H. Liu et al., Efficient nitrate conversion to ammonia on f-block single-atom/metal oxide heterostructure via local electron-deficiency modulation. ACS Nano 16(9), 15297–15309 (2022). https://doi.org/10.1021/acsnano.2c06747
Y.T. Wang, H.J. Li, W. Zhou, X. Zhang, B. Zhang et al., Structurally disordered RuO2 nanosheets with rich oxygen vacancies for enhanced nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. 61(19), e202202604 (2022). https://doi.org/10.1002/anie.202202604
J. Li, G.M. Zhan, J.H. Yang, F.J. Quan, C.L. Mao et al., Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142(15), 7036–7046 (2020). https://doi.org/10.1021/jacs.0c00418
H.B. Yin, Z. Chen, S.C. Xiong, J.J. Chen, C.Z. Wang et al., Alloying effect-induced electron polarization drives nitrate electroreduction to ammonia. Chem. Catal. 1(5), 1088–1103 (2021). https://doi.org/10.1016/j.checat.2021.08.014