Synthesis of Nanostructured Copper-doped Titania and Its Properties
Corresponding Author: Oman Zuas
Nano-Micro Letters,
Vol. 5 No. 1 (2013), Article Number: 26-33
Abstract
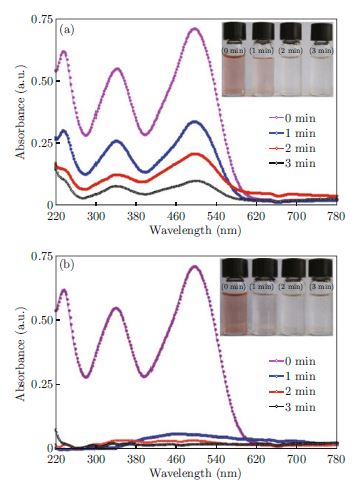
Nanostructured pure-TiO2 and Cu3%-TiO2 were successfully synthesized via co-precipitation method. The X-ray diffraction (XRD) result proves that the synthesized sample were predominantly in anatase phase with size in the range of 8∼16 nm, which are in good agreement with the transmission electron microscopy data. Owing to doping of copper, not only did the thermal stability of the TiO2 decrease, but also a significant decrease in its particle size and a shift of the adsorption edge to a higher wavelength region appear. The activity of both pure-TiO2 and Cu3%-doped TiO2 was tested to study their ability to decolorize congo red (CR) dye in aqueous solution. We observed that the CR dye was decolorized faster by Cu3%-TiO2 than pure-TiO2. Results of this study demonstrate a potential application of the synthesized sample for decolorizing dye pollutants from aqueous waste.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- A. Fujishima and K. Honda, “Electrochemical photolysis of water at a semiconductor electrode”, Nature 238, 37–38 (1972). http://dx.doi.org/10.1038/238037a0
- O. Carp, C. L. Huisman and A. Reller, “Photoinduced reactivity of titanium dioxide”, Prog. Solid State Ch. 32(1–2), 33–177 (2004). http://dx.doi.org/10.1016/j.progsolidstchem.2004.08.001
- G. R. Dey, “Chemical reduction of CO2 to different products during photo catalytic reaction on TiO2 under diverse conditions: An overview”, J. Nat. Gas Chem. 16(3), 217–226 (2007). http://dx.doi.org/10. 1016/S1003-9953(07)60052-8
- K. Koč’i, L. Obalová and Z. Lacný “Photocatalytic reduction of CO2 over TiO2 based catalysts”, Chem. Pap. 62(1), 1–9 (2008). http://dx.doi.org/10.2478/s11696-007-0072-x
- A. L. Linsebigler, G. Lu and J. J. T. Yates, “Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results”, Chem. Rev. 95(3), 735–758 (1995). http://dx.doi.org/10.1021/cr00035a013
- J. Cyviene, D. Milcius and G. Laukaitis, “Porosity evaluation of TiO2 thin films deposited using pulsed DC-magnetron sputtering”, Mat. Sci. (Medziagotyra), 15(2), 103–107 (2009).
- B. B. F. Mirjalili and A. Akbari, “Nano-TiO2: An ecofriendly alternative for the synthesis of quinoxalines”, Chinese Chem. Lett. 22(6), 753–756 (2011). http://dx.doi.org/10.1016/j.cclet.2010.12.016
- M. C. Wu, A. Sápi, A. Avila, M. Szabó J. Hiltunen, M. Huuhtanen, G. Tóth, A. Kukovecz, A. Kónya, R. Keiski, W. F. Su, H. Jantunen and K. Kordás, “Enhanced photocatalytic activity of TiO2 nanofibers and their flexible composite films: Decomposition of organic dyes and efficient H2 generation from ethanol-water mixtures”, Nano Res. 4(4), 360–369 (2011). http://dx.doi.org/10.1007/s12274-010-0090-9
- B. Reddy, G. Reddy, K. Rao, I. Ganesh and J. Ferreira, “Characterization and photocatalytic activity of TiO2- MxOy (MxOy=SiO2, Al2O3, and ZrO2) mixed oxides synthesized by microwave-induced solution combus- tion technique”, Mater. Sci. 44(18), 4874–4882 (2009). http://dx.doi.org/10.1007/s10853-009-3743-x
- S. Yang, W. Zhua, J. Wang and Z. Chen, “Catalytic wet air oxidation of phenol over CeO2-TiO2 catalyst in the batch reactor and the packed-bed reactor”, J. Hazard. Mater. 153(3), 1248–1253 (2008). http://dx.doi.org/10.1016/j.jhazmat.2007.09.084
- J. Bandara, C. P. K. Udawatta and C. S. K. Rajapakse, “Highly stable CuO incorporated TiO2 catalyst for photocatalytic hydrogen production from H2O”, Photochem. & Photobiol.: Sciences 4(11), 857–861 (2005). http://dx.doi.org/10.1039/B507816D
- X. Zhang and L. Lei, “Preparation of photocatalytic Fe2O3-TiO2 coatings in one step by metal organic chemical vapor deposition”, Appl. Surf. Sci. 254(8), 2406–2412 (2008). http://dx.doi.org/10.1016/j.apsusc.2007.09.067
- L. Shi, C. Li, H. Gu and D. Fang, “Morphology and properties of ultrafine SnO2-TiO2 coupled semiconductor particles”, Mater. Chem. Phys. 62(1), 62–67 (2000). http://dx.doi.org/10.1016/S0254-0584 (99)00171-6
- T. Mishra, J. Hait, N. Aman, M. Gunjan, B. Mahato and R. K. Jana, “Surfactant mediated synthesis of spherical binary oxides photocatalytic with enhanced activity in visible light”, Colloid Interf. Sci. 327(2), 377–383 (2008). http://dx.doi.org/10.1016/j.jcis.2008.08.040
- J. He, Q. Z. Cai, Q. Luo, D. Q. Zhang, T. T. Tang and Y. F. Jiang, “Photocatalytic removal of methyl orange in an aqueous solution by a WO3/TiO2 composite film”, Korean J. Chem.Eng. 27(2), 435–438 (2010). http://dx.doi.org/10.1007/s11814-010-0080-3
- Y. Zhao, C. Li, X. Liu, F. Gu, H. L. Du and L. Shi, “Zn-doped TiO2 nanoparticles with high photocatalytic activity synthesized by hydrogen-oxygen diffusion flame”, Appl. Catal. B-Environ. 79(3), 208–215 (2008). http://dx.doi.org/10.1016/j.apcatb.2007.09.044
- Y. Liu, H. Zhou, J. Li, H. Chen, D. Li, B. Zhou and W. Cai, “Enhanced photoelectrochemical properties of Cu2O-loaded short TiO2 nanotube array electrode prepared by sonoelectrochemical deposition”, Nano-Micro Lett. 2(4), 277–284 (2010). http://dx.doi.org/10.3786/nml.v2i4.p277-284
- I. H. Tseng, W.-C. Chang and J. C. S. Wu, “Photoreduction of CO2 using sol-gel derived titania and titania-supported copper catalysts”, Appl. Catal. B: Environ. 37(1), 37–48 (2002). http://dx.doi.org/10.1016/S0926-3373(01)00322-8
- R. Lopez, R. Goez and M. E. Llanos, “Photophysical and photocatalytic properties of nanosized copperdoped titania sol-gel catalysts”, Catal. Today 148(1–2), 103–108 (2009). http://dx.doi.org/10.1016/j.cattod.2009.04.001
- I. H. Tseng, J. C. S. Wu and H.-Y. Chou, “Effects of sol-gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction”, J. Catalysis, 221(2), 432–440 (2004). http://dx.doi.org/10.1016/j.jcat.2003.09.002
- L. S. Yoong, F. K. Chong and B. K. Dutta, “Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light”, Energy 34(10), 1652–1661, (2009). http://dx.doi.org/10.1016/j.energy.2009.07.024
- F. Boccuzzi, A. Chiorino, M. Manzoli, D. Andreeva, T. Tabakova, V. Ilieva and L. Iadakiev, “Gold, silver and copper catalysts supported on TiO2 for pure hydrogen production”, Catal. Today 75(1-4), 169–175 (2002). http://dx.doi.org/10.1016/S0920-5861(02)00060-3
- B. Zhu, Q. Guo, X. Huang, S. Wang, S. Zhang, S. Wu and W. Huang, “Characterization and catalytic performance of TiO2 nanotubes-supported gold and copper particles”, Mol. Catal. A: Chem. 249(1–2), 211–217 (2006). http://dx.doi.org/10.1016/j.molcata.2006.01.013
- B. Zhu, X. Zhang, S. Wang, S. Zhang, S. Wu and W. Huang, “Synthesis and catalytic performance of TiO2 nanotubes-supported copper oxide for low-temperature CO oxidation”, Micropor. Mesopor. Mat. 102(1–3), 333–336 (2007). http://dx.doi.org/10.1016/j.micromeso.2006.11.028
- T. Sato and M. Taya, “Copper-aided photosterilization of microbial cells on TiO2 film under irradiation from a white light fluorescent lamp”, Biochem. Eng. 30(2), 199–204 (2006). http://dx.doi.org/10.1016/j.bej.2006.04.002
- M. B. Fisher, D. A. Keane, P. Fernández-Ibánez, J. Colreavy, S. J. Hinder, K. G. McGuigan and S. C. Pilla, “Nitrogen and copper doped solar light ac- tive TiO2 photocatalysts for water decontamination”, Appl. Catal. B: Environ. 130-131, 8–13 (2013). http://dx.doi.org/10.1016/j.apcatb.2012.10.013
- B. Xin, P. Wang, D. Ding, J. Liu, Z. Ren and H. Fu, “Effect of surface species on Cu-TiO2 photocatalytic activity”, Appl. Surf. Sci. 254(9), 2569–2574 (2008). http://dx.doi.org/10.1016/j.apsusc.2007.09.002
- S. Watanabe, X. Ma and C. Song, “Selective sulfur removal from liquid hydrocarbon over regenerable CeO2-TiO2 adsorbent for fuel cell application”, ACS: Div. Fuel Chem. 49(2), 511–513 (2004).
- B. B. Kefi, L. L. E. Atracheb, H. Kochkar anfd A. Ghorbel, “TiO2 nanotubes as solid-phase extraction adsorbent for the determination of polycyclic aromatic hydrocarbons in environmental water samples”, J. Environ. Sci. 23(5), 860–867 (2011). http://dx.doi.org/10.1016/S1001-0742 (10)60481-0
- Y. Luo and D. Li, “Experimental study of nanometer TiO2 for use as an adsorbent for SO2 removal”, Dev. Chem. Eng. Min. Process 10(3–4), 443–457 (2002). http://dx.doi.org/10.1002/apj.5500100414
- S. Deng, Z. Lia, J. Huang and G. Yua, “Preparation, characterization and application of a Ce-Ti oxide adsorbent for enhanced removal of arsenate from water”, J. Hazard Mater. 179(1–3), 1014–1021 (2010). http://dx.doi.org/10.1016/j.jhazmat.2010.03.106
- S. J. Kim, E. G. Lee, S. D. Park, C. J. Jeon, Y. H. Cho, C. K. Rhee and W. W. Kim, “Photocatalytic effects of rutile phase TiO2 ultrafine powder with high specific surface area obtained by a homogeneous precipitation process at low temperatures”, Sol-Gel Sci. Tech. 22(1–2), 63–74 (2001). http://dx.doi.org/10.1023/A:1011264320138
- O. Zuas, H. Budiman and N. Hamim, “Anatase TiO2 and mixed M-Anatase TiO2 (M = CeO2 or ZrO2) nano powder: synthesis and characterization”, I. J. Nano Dimens. 4(1), 11–18 (2013) In press.
- S. Martini and M. Herrera, “X-ray diffraction and crystal size”, J. Am. Oil Chem. Soc. 79(3), 315–316 (2002). http://dx.doi.org/10.1007/s11746-002-0480-z
- Y. Masuda and K. Kato, “Synthesis and phase transformation of TiO2 nano-crystal in aqueous solutions”, Ceram. Soc. Jpn. 117(1363), 373–376 (2009). http://dx.doi.org/10.2109/jcersj2.117.373
- N. Sasirekha, S. J. S. Basha and K. Shanthi, “Photo catalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide”, Appl. Catal. B: Environ. 62(1), 169–180 (2006). http://dx.doi.org/10.1016/j.apcatb.2005.07.009
- L. Ge, M. Xu, M. Sun and H. Fang, “Low-temperature synthesis of photocatalytic TiO2 thin film from aqueous anatase precursor sols”, J. Sol-Gel Sci. Techn. 38(1), 47–53 (2006). http://dx.doi.org/10.1007/s10971-006-6009-y
- S. R. Dhage, S. P. Gaikwad anfd V. Ravi, “Synthesis of nanocrystalline TiO2 by tartarate gel method”, B. Mater. Sci. 27(6), 487–489 (2004). http://dx.doi.org/10.1007/BF02707273
- O. Vázquez-Cuchillo, A. Cruz-López, L. M. Bautista-Carrillo, A. Bautista-Hernández, L. M. Torres Martínez and S. W. Lee, “Synthesis of TiO2 using different hydrolysis catalysts and doped with Zn for efficient degradation of aqueous phase pollutants under UV light”, Chem. Intermediet. 36(1), 103–113 (2010). http://dx.doi.org/10.1007/s11164-010-0119-4
- R. López, R. Gómez andf M. E. Llanos, “Photophysical and photocatalytic properties of nanosized copperdoped titania sol-gel catalysts”, Catal. Today 148(1–2), 103–108 (2009). http://dx.doi.org/10.1016/j.cattod.2009.04.001
- M. Sabzi, S. M. Mirabedini, J. Zohuriaan-Mehr and M. Atai, “Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating”, Progr. Org. Coating. 65(2), 222–228 (2009). http://dx.doi.org/10.1016/j.porgcoat.2008.11.006
References
A. Fujishima and K. Honda, “Electrochemical photolysis of water at a semiconductor electrode”, Nature 238, 37–38 (1972). http://dx.doi.org/10.1038/238037a0
O. Carp, C. L. Huisman and A. Reller, “Photoinduced reactivity of titanium dioxide”, Prog. Solid State Ch. 32(1–2), 33–177 (2004). http://dx.doi.org/10.1016/j.progsolidstchem.2004.08.001
G. R. Dey, “Chemical reduction of CO2 to different products during photo catalytic reaction on TiO2 under diverse conditions: An overview”, J. Nat. Gas Chem. 16(3), 217–226 (2007). http://dx.doi.org/10. 1016/S1003-9953(07)60052-8
K. Koč’i, L. Obalová and Z. Lacný “Photocatalytic reduction of CO2 over TiO2 based catalysts”, Chem. Pap. 62(1), 1–9 (2008). http://dx.doi.org/10.2478/s11696-007-0072-x
A. L. Linsebigler, G. Lu and J. J. T. Yates, “Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results”, Chem. Rev. 95(3), 735–758 (1995). http://dx.doi.org/10.1021/cr00035a013
J. Cyviene, D. Milcius and G. Laukaitis, “Porosity evaluation of TiO2 thin films deposited using pulsed DC-magnetron sputtering”, Mat. Sci. (Medziagotyra), 15(2), 103–107 (2009).
B. B. F. Mirjalili and A. Akbari, “Nano-TiO2: An ecofriendly alternative for the synthesis of quinoxalines”, Chinese Chem. Lett. 22(6), 753–756 (2011). http://dx.doi.org/10.1016/j.cclet.2010.12.016
M. C. Wu, A. Sápi, A. Avila, M. Szabó J. Hiltunen, M. Huuhtanen, G. Tóth, A. Kukovecz, A. Kónya, R. Keiski, W. F. Su, H. Jantunen and K. Kordás, “Enhanced photocatalytic activity of TiO2 nanofibers and their flexible composite films: Decomposition of organic dyes and efficient H2 generation from ethanol-water mixtures”, Nano Res. 4(4), 360–369 (2011). http://dx.doi.org/10.1007/s12274-010-0090-9
B. Reddy, G. Reddy, K. Rao, I. Ganesh and J. Ferreira, “Characterization and photocatalytic activity of TiO2- MxOy (MxOy=SiO2, Al2O3, and ZrO2) mixed oxides synthesized by microwave-induced solution combus- tion technique”, Mater. Sci. 44(18), 4874–4882 (2009). http://dx.doi.org/10.1007/s10853-009-3743-x
S. Yang, W. Zhua, J. Wang and Z. Chen, “Catalytic wet air oxidation of phenol over CeO2-TiO2 catalyst in the batch reactor and the packed-bed reactor”, J. Hazard. Mater. 153(3), 1248–1253 (2008). http://dx.doi.org/10.1016/j.jhazmat.2007.09.084
J. Bandara, C. P. K. Udawatta and C. S. K. Rajapakse, “Highly stable CuO incorporated TiO2 catalyst for photocatalytic hydrogen production from H2O”, Photochem. & Photobiol.: Sciences 4(11), 857–861 (2005). http://dx.doi.org/10.1039/B507816D
X. Zhang and L. Lei, “Preparation of photocatalytic Fe2O3-TiO2 coatings in one step by metal organic chemical vapor deposition”, Appl. Surf. Sci. 254(8), 2406–2412 (2008). http://dx.doi.org/10.1016/j.apsusc.2007.09.067
L. Shi, C. Li, H. Gu and D. Fang, “Morphology and properties of ultrafine SnO2-TiO2 coupled semiconductor particles”, Mater. Chem. Phys. 62(1), 62–67 (2000). http://dx.doi.org/10.1016/S0254-0584 (99)00171-6
T. Mishra, J. Hait, N. Aman, M. Gunjan, B. Mahato and R. K. Jana, “Surfactant mediated synthesis of spherical binary oxides photocatalytic with enhanced activity in visible light”, Colloid Interf. Sci. 327(2), 377–383 (2008). http://dx.doi.org/10.1016/j.jcis.2008.08.040
J. He, Q. Z. Cai, Q. Luo, D. Q. Zhang, T. T. Tang and Y. F. Jiang, “Photocatalytic removal of methyl orange in an aqueous solution by a WO3/TiO2 composite film”, Korean J. Chem.Eng. 27(2), 435–438 (2010). http://dx.doi.org/10.1007/s11814-010-0080-3
Y. Zhao, C. Li, X. Liu, F. Gu, H. L. Du and L. Shi, “Zn-doped TiO2 nanoparticles with high photocatalytic activity synthesized by hydrogen-oxygen diffusion flame”, Appl. Catal. B-Environ. 79(3), 208–215 (2008). http://dx.doi.org/10.1016/j.apcatb.2007.09.044
Y. Liu, H. Zhou, J. Li, H. Chen, D. Li, B. Zhou and W. Cai, “Enhanced photoelectrochemical properties of Cu2O-loaded short TiO2 nanotube array electrode prepared by sonoelectrochemical deposition”, Nano-Micro Lett. 2(4), 277–284 (2010). http://dx.doi.org/10.3786/nml.v2i4.p277-284
I. H. Tseng, W.-C. Chang and J. C. S. Wu, “Photoreduction of CO2 using sol-gel derived titania and titania-supported copper catalysts”, Appl. Catal. B: Environ. 37(1), 37–48 (2002). http://dx.doi.org/10.1016/S0926-3373(01)00322-8
R. Lopez, R. Goez and M. E. Llanos, “Photophysical and photocatalytic properties of nanosized copperdoped titania sol-gel catalysts”, Catal. Today 148(1–2), 103–108 (2009). http://dx.doi.org/10.1016/j.cattod.2009.04.001
I. H. Tseng, J. C. S. Wu and H.-Y. Chou, “Effects of sol-gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction”, J. Catalysis, 221(2), 432–440 (2004). http://dx.doi.org/10.1016/j.jcat.2003.09.002
L. S. Yoong, F. K. Chong and B. K. Dutta, “Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light”, Energy 34(10), 1652–1661, (2009). http://dx.doi.org/10.1016/j.energy.2009.07.024
F. Boccuzzi, A. Chiorino, M. Manzoli, D. Andreeva, T. Tabakova, V. Ilieva and L. Iadakiev, “Gold, silver and copper catalysts supported on TiO2 for pure hydrogen production”, Catal. Today 75(1-4), 169–175 (2002). http://dx.doi.org/10.1016/S0920-5861(02)00060-3
B. Zhu, Q. Guo, X. Huang, S. Wang, S. Zhang, S. Wu and W. Huang, “Characterization and catalytic performance of TiO2 nanotubes-supported gold and copper particles”, Mol. Catal. A: Chem. 249(1–2), 211–217 (2006). http://dx.doi.org/10.1016/j.molcata.2006.01.013
B. Zhu, X. Zhang, S. Wang, S. Zhang, S. Wu and W. Huang, “Synthesis and catalytic performance of TiO2 nanotubes-supported copper oxide for low-temperature CO oxidation”, Micropor. Mesopor. Mat. 102(1–3), 333–336 (2007). http://dx.doi.org/10.1016/j.micromeso.2006.11.028
T. Sato and M. Taya, “Copper-aided photosterilization of microbial cells on TiO2 film under irradiation from a white light fluorescent lamp”, Biochem. Eng. 30(2), 199–204 (2006). http://dx.doi.org/10.1016/j.bej.2006.04.002
M. B. Fisher, D. A. Keane, P. Fernández-Ibánez, J. Colreavy, S. J. Hinder, K. G. McGuigan and S. C. Pilla, “Nitrogen and copper doped solar light ac- tive TiO2 photocatalysts for water decontamination”, Appl. Catal. B: Environ. 130-131, 8–13 (2013). http://dx.doi.org/10.1016/j.apcatb.2012.10.013
B. Xin, P. Wang, D. Ding, J. Liu, Z. Ren and H. Fu, “Effect of surface species on Cu-TiO2 photocatalytic activity”, Appl. Surf. Sci. 254(9), 2569–2574 (2008). http://dx.doi.org/10.1016/j.apsusc.2007.09.002
S. Watanabe, X. Ma and C. Song, “Selective sulfur removal from liquid hydrocarbon over regenerable CeO2-TiO2 adsorbent for fuel cell application”, ACS: Div. Fuel Chem. 49(2), 511–513 (2004).
B. B. Kefi, L. L. E. Atracheb, H. Kochkar anfd A. Ghorbel, “TiO2 nanotubes as solid-phase extraction adsorbent for the determination of polycyclic aromatic hydrocarbons in environmental water samples”, J. Environ. Sci. 23(5), 860–867 (2011). http://dx.doi.org/10.1016/S1001-0742 (10)60481-0
Y. Luo and D. Li, “Experimental study of nanometer TiO2 for use as an adsorbent for SO2 removal”, Dev. Chem. Eng. Min. Process 10(3–4), 443–457 (2002). http://dx.doi.org/10.1002/apj.5500100414
S. Deng, Z. Lia, J. Huang and G. Yua, “Preparation, characterization and application of a Ce-Ti oxide adsorbent for enhanced removal of arsenate from water”, J. Hazard Mater. 179(1–3), 1014–1021 (2010). http://dx.doi.org/10.1016/j.jhazmat.2010.03.106
S. J. Kim, E. G. Lee, S. D. Park, C. J. Jeon, Y. H. Cho, C. K. Rhee and W. W. Kim, “Photocatalytic effects of rutile phase TiO2 ultrafine powder with high specific surface area obtained by a homogeneous precipitation process at low temperatures”, Sol-Gel Sci. Tech. 22(1–2), 63–74 (2001). http://dx.doi.org/10.1023/A:1011264320138
O. Zuas, H. Budiman and N. Hamim, “Anatase TiO2 and mixed M-Anatase TiO2 (M = CeO2 or ZrO2) nano powder: synthesis and characterization”, I. J. Nano Dimens. 4(1), 11–18 (2013) In press.
S. Martini and M. Herrera, “X-ray diffraction and crystal size”, J. Am. Oil Chem. Soc. 79(3), 315–316 (2002). http://dx.doi.org/10.1007/s11746-002-0480-z
Y. Masuda and K. Kato, “Synthesis and phase transformation of TiO2 nano-crystal in aqueous solutions”, Ceram. Soc. Jpn. 117(1363), 373–376 (2009). http://dx.doi.org/10.2109/jcersj2.117.373
N. Sasirekha, S. J. S. Basha and K. Shanthi, “Photo catalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide”, Appl. Catal. B: Environ. 62(1), 169–180 (2006). http://dx.doi.org/10.1016/j.apcatb.2005.07.009
L. Ge, M. Xu, M. Sun and H. Fang, “Low-temperature synthesis of photocatalytic TiO2 thin film from aqueous anatase precursor sols”, J. Sol-Gel Sci. Techn. 38(1), 47–53 (2006). http://dx.doi.org/10.1007/s10971-006-6009-y
S. R. Dhage, S. P. Gaikwad anfd V. Ravi, “Synthesis of nanocrystalline TiO2 by tartarate gel method”, B. Mater. Sci. 27(6), 487–489 (2004). http://dx.doi.org/10.1007/BF02707273
O. Vázquez-Cuchillo, A. Cruz-López, L. M. Bautista-Carrillo, A. Bautista-Hernández, L. M. Torres Martínez and S. W. Lee, “Synthesis of TiO2 using different hydrolysis catalysts and doped with Zn for efficient degradation of aqueous phase pollutants under UV light”, Chem. Intermediet. 36(1), 103–113 (2010). http://dx.doi.org/10.1007/s11164-010-0119-4
R. López, R. Gómez andf M. E. Llanos, “Photophysical and photocatalytic properties of nanosized copperdoped titania sol-gel catalysts”, Catal. Today 148(1–2), 103–108 (2009). http://dx.doi.org/10.1016/j.cattod.2009.04.001
M. Sabzi, S. M. Mirabedini, J. Zohuriaan-Mehr and M. Atai, “Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating”, Progr. Org. Coating. 65(2), 222–228 (2009). http://dx.doi.org/10.1016/j.porgcoat.2008.11.006