Highly Efficient Adsorption of Copper Ions by a PVP-Reduced Graphene Oxide Based On a New Adsorptions Mechanism
Corresponding Author: Yu Gu
Nano-Micro Letters,
Vol. 6 No. 1 (2014), Article Number: 80-87
Abstract
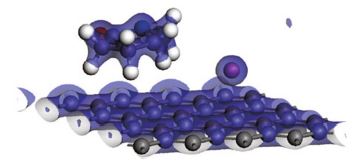
Polyvinylpyrrolidone-reduced graphene oxide was prepared by modified hummers method and was used as adsorbent for removing Cu ions from wastewater. The effects of contact time and ions concentration on adsorption capacity were examined. The maximum adsorption capacity of 1689 mg/g was observed at an initial pH value of 3.5 after agitating for 10 min. It was demonstrated that polyvinylpyrrolidone-reduced graphene oxide had a huge adsorption capacity for Cu ions, which was 10 times higher than maximal value reported in previous works. The adsorption mechanism was also discussed by density functional theory. It demonstrates that Cu ions are attracted to surface of reduced graphene oxide by C atoms in reduced graphene oxide modified by polyvinylpyrrolidone through physisorption processes, which may be responsible for the higher adsorption capacity. Our results suggest that polyvinylpyrrolidone-reduced graphene oxide is an effective adsorbent for removing Cu ions in wastewater. It also provides a new way to improve the adsorption capacity of reduced graphene oxide for dealing with the heavy metal ion in wastewater.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Y. F. Zhang, Q. Chen, Z. D. Wang, G. Q. Zhang and Y. J. Ge, “Preparation of Cr hard coatings by ion beam assisted electron beam vapor deposition on Ni and Cu substrates”, Surf. Coat. Tech. 201(9–11), 5190–1592 (2007). http://dx.doi.org/10.1016/j.surfcoat.2006.07.138
- T. S. Anirudhan, S. Jalajamony and S. S. Sreekumari, “Adsorptive removal of Cu(II) ions from aqueous media onto 4-ethyl thiosemicarbazide intercalated organophilic calcined hydrotalcite”, J. Chem. Eng. Data 58(1), 24–31 (2013). http://dx.doi.org/10.1021/je300536u
- F. Massimiliano, N. Biagio, T. Gelsomina and d’. Luca, “An environmental friendly cycle for Cr(III) removal and recovery from tannery wastewater”, J. Environ. Manage. 117, 1–6 (2013). http://dx.doi.org/10.1016/j.jenvman.2012.12.012
- T. B. Budak, “Removal of heavy metals from wastewater using synthetic ion exchange resin”, Asian J. Chem. 25(8), 4207–4210 (2013).
- E-S. Z. El-Ashtoukhy, T. M. Zewail and N. K. Amin, “Removal of heavy metal ions from aqueous solution by electrocoagulation using a horizontal expanded Al anode”, Desalin. Water Treat. 20(1–3), 72–79 (2010). http://dx.doi.org/10.5004/dwt.2010.1127
- R. M. Huang, J. Y. He, J. Zhao, Q. Luo and C. M. Huang, “Fenton-biological treatment of reverse osmosis membrane concentrate from a metal plating wastewater recycle system”, Environ Technol. 32(5), 515–522 (2010). http://dx.doi.org/10.1080/09593330.2010.504747
- H. Z. Mousavi and A. Asghari, “Removal of heavy metal ions in wastewater by Semnan natural zeolite”, Asian J. Chem. 21(4), 2881–2886 (2009).
- Y. F. Lin, H. W. Chen, K. L. Lin, B. Y. Chen and C. Chiou, “Application of magnetic particles modified with amino groups to adsorb copper ions in aqueous solution”, J. Environ. Sci. 23(1), 44–50 (2011). http://dx.doi.org/10.1016/S1001-0742 (10)60371-3
- A. K. Mark, “The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers”, Colloid. Surface. A 138(1), 11–20 (1998). http://dx.doi.org/10.1016/S0927-7757(97)00078-2
- X. J. Hu, Y. G. Liu, H. Wang, A. W. Chen, G. M. Zeng, S. M. Liu, Y. M. Guo, X. Hu, T. T. Li, Y. Q. Wang, L. Zhou and S. H. Liu, “Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite”, Sep. Purif. Technol. 108, 189–195 (2013). http://dx.doi.org/10.1016/j.seppur.2013.02.011
- J. Li, S. W. Zhang, C.L. Chen, G.X. Zhao, X. Yang, J. X. Li and X. K. Wang, “Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles”, ACS Appl. Mater. Inter. 4(9), 4991–5000 (2012). http://dx.doi.org/10.1021/am301358b
- A. Roy and J. T. Bhattacharya, “Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes”, Chem. Eng. J. 211, 493–500 (2012). http://dx.doi.org/10.1016/j.cej.2012.09.097
- D. K. Venkata Ramana, Jae Su Yu and K. Seshaiah, “Silver nanoparticles deposited multiwalled carbon nanotubes for removal of Cu(II) and Cd(II) from water: Surface, kinetic, equilibrium, and thermal adsorption properties”, Chem. Eng. J. 223, 806–815 (2013). http://dx.doi.org/10.1016/j.cej.2013.03.001
- J. Gao, F. Q. Liu, P. P. Ling, J. T. Lei, L. J. Li, C. H. Li and A. M. Li, “High efficient removal of Cu(II) by a chelating resin from strong acidic solutions: Complex formation and DFT certification”, Chem. Eng. J. 222, 240–247 (2013). http://dx.doi.org/10.1016/j.cej.2013.02.055
- K. G. Bhattacharyya and S. S. Gupta, “Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics”, Desalination 272(1–3), 66–75 (2011). http://dx.doi.org/10.1016/j.desal.2011.01.001
- W. Q. Wu, Y. Yang, H. H. Zhou, T. T. Ye, Z. Y. Huang, R. Liu and Y. F. Kuang, “Highly efficient removal of Cu(II) from aqueous solution by using graphene oxide”, Water Air Soil. Poll. 224(1), 1372 (1–8) (2013). http://dx.doi.org/10.1007/s11270-012-1372-5
- X. W. Wu, H. W. Ma, L. T. Zhang and F. J. Wang, “Adsorption properties and mechanism of mesoporous adsorbents prepared with fly ash for removal of Cu(II) in aqueous solution”, Appl. Surf. Sci. 261, 902–907 (2012). http://dx.doi.org/10.1016/j.apsusc.2012.08.122
- D. Li, M. B. MüLler, S. Gilje, R. B. Kaner and G. G. Wallace, “Processable aqueous dispersions of graphene nanosheets”, Nat. Nanotechnol. 3(2), 101–105 (2008). http://dx.doi.org/10.1038/nnano.2007.451
- L. H. Aia, C. Zhang and Z. Chen, “Removal of methylene blue from aqueous solution by asolvothermal synthesized graphene/magnetite composite”, J. Hazard. Mater. 192(3), 1515–1524 (2011). http://dx.doi.org/10.1016/j.jhazmat.2011.06.068
- T. S. Sreeprasad, S. S. Gupt, S. M. Maliyekkal and T. Pradeep, “Immobilized graphene-based composite from asphalt: Facile synthesis and application in water purification”, J. Hazard. Mater. 246, 213–220 (2013). http://dx.doi.org/10.1016/j.jhazmat.2012.12.022
- K. C. Kemp, H. Seema, M. Saleh and N. H. Le, “Environmental applications using graphene composites: water remediation and gas adsorption”. Nanoscale 5(8), 3149–3171 (2013). http://dx.doi.org/10.1039/c3nr33708a
- V. Georgakilas, M. Otyepka and A. B. Bourlinos, “Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications”, Chem. Rev. 112(11), 6156–6214 (2012). http://dx.doi.org/10.1021/cr3000412
- V. Chandra, J. Park and Y. Chun, “Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal”, ACS Nano 4(7), 3979–3986 (2010). http://dx.doi.org/10.1021/nn1008897
- Z. H. Huang, X. Y. Heng, W. Lv, M. Wang, Q. H. Yang and F. Y. Kang, “Adsorption of Lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets”, Langmuir 27(12), 7558–7562 (2011). http://dx.doi.org/10.1021/la200606r
- Y. W. Zhang, H. L. Ma, J. Peng, M. L. Zhai and Z. Z. Yu, “Cr(VI) removal from aqueous solution using chemically reduced and functionalized graphene oxide”, J. Mater. Sci. 48(5), 1883–1889 (2013). http://dx.doi.org/10.1007/s10853-012-6951-8
- V. Chandra and K. S. Kim, “Highly selective adsorption of Hg2+ by a polypyrrole-reduced graphene oxide composite”, Chem. Commun. 47(13), 3942–3944 (2011). http://dx.doi.org/10.1039/c1cc00005e
- K. Moon, J. H. Lee, R. S. Ruoff and H. Lee, “Reduced graphene oxide by chemical graphitization”, Nat. commun. 1, 73(1–6) (2010). http://dx.doi.org/10.1038/ncomms1067
- Q. L Du, M. B. Zheng, L. F. Zhang, Y. W. Wang, J. H. Chen, L. P. Xue, W. J. Dai, G. B. Ji and J. M. Cao, “Preparation of functionalized graphene sheets by a low-temperature thermal exfoliation approach and their electrochemical supercapacitive behaviors”, Electrochim. Acta 55(12), 3897–3903 (2010). http://dx.doi.org/10.1016/j.electacta.2010.01.089
- D. F. Han, C. S. Shan, L. P. Guo, L. Niu and D. X. Han, “Electro-oxidation of ascorbic acid on PVP-stabilized graphene electrode”, Chem. Res. Chinese U. 26(2), 287–290 (2010).
- Q. F. Lu, J. Yu, J. Z. Gao, W. Yang and Y. Li, “Glowdischarge Electrolysis Plasma Induced Synthesis of Polyvinylpyrrolidone/Acrylic Acid Hydrogel and its Adsorption Properties for Heavy-metal Ions”, Plasma Process. Polym. 8(9), 803–814 (2011). http://dx.doi.org/10.1002/ppap.201000144
- J. H. Zhao, W. Z. Yuan, A. H. Xu, F. Ai, Y. W. Lu and Y. M. Zhang, “Perfluorinated sulfonic acid ionomer/poly(N-vinylpyrrolidone) nanofiber membranes: Electrospinning fabrication, water stability, and metal ion removal applications”, React. Funct. Polym. 71(11), 1102–1109 (2011). http://dx.doi.org/10.1016/j.reactfunctpolym.2011.08.007
- Y. H. Zhang, K. G. Zhou, K. F. Xie, H. L. Zhang, Y. Peng and C. W. Wang, “Tuning the magnetic and transport properties of metal adsorbed graphene by co-adsorption with 1,2-dichlorobenzene”, Phys. Chem. Chem. Phys 14(33), 11626–11632 (2012). http://dx.doi.org/10.1039/c2cp41370a
References
Y. F. Zhang, Q. Chen, Z. D. Wang, G. Q. Zhang and Y. J. Ge, “Preparation of Cr hard coatings by ion beam assisted electron beam vapor deposition on Ni and Cu substrates”, Surf. Coat. Tech. 201(9–11), 5190–1592 (2007). http://dx.doi.org/10.1016/j.surfcoat.2006.07.138
T. S. Anirudhan, S. Jalajamony and S. S. Sreekumari, “Adsorptive removal of Cu(II) ions from aqueous media onto 4-ethyl thiosemicarbazide intercalated organophilic calcined hydrotalcite”, J. Chem. Eng. Data 58(1), 24–31 (2013). http://dx.doi.org/10.1021/je300536u
F. Massimiliano, N. Biagio, T. Gelsomina and d’. Luca, “An environmental friendly cycle for Cr(III) removal and recovery from tannery wastewater”, J. Environ. Manage. 117, 1–6 (2013). http://dx.doi.org/10.1016/j.jenvman.2012.12.012
T. B. Budak, “Removal of heavy metals from wastewater using synthetic ion exchange resin”, Asian J. Chem. 25(8), 4207–4210 (2013).
E-S. Z. El-Ashtoukhy, T. M. Zewail and N. K. Amin, “Removal of heavy metal ions from aqueous solution by electrocoagulation using a horizontal expanded Al anode”, Desalin. Water Treat. 20(1–3), 72–79 (2010). http://dx.doi.org/10.5004/dwt.2010.1127
R. M. Huang, J. Y. He, J. Zhao, Q. Luo and C. M. Huang, “Fenton-biological treatment of reverse osmosis membrane concentrate from a metal plating wastewater recycle system”, Environ Technol. 32(5), 515–522 (2010). http://dx.doi.org/10.1080/09593330.2010.504747
H. Z. Mousavi and A. Asghari, “Removal of heavy metal ions in wastewater by Semnan natural zeolite”, Asian J. Chem. 21(4), 2881–2886 (2009).
Y. F. Lin, H. W. Chen, K. L. Lin, B. Y. Chen and C. Chiou, “Application of magnetic particles modified with amino groups to adsorb copper ions in aqueous solution”, J. Environ. Sci. 23(1), 44–50 (2011). http://dx.doi.org/10.1016/S1001-0742 (10)60371-3
A. K. Mark, “The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers”, Colloid. Surface. A 138(1), 11–20 (1998). http://dx.doi.org/10.1016/S0927-7757(97)00078-2
X. J. Hu, Y. G. Liu, H. Wang, A. W. Chen, G. M. Zeng, S. M. Liu, Y. M. Guo, X. Hu, T. T. Li, Y. Q. Wang, L. Zhou and S. H. Liu, “Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite”, Sep. Purif. Technol. 108, 189–195 (2013). http://dx.doi.org/10.1016/j.seppur.2013.02.011
J. Li, S. W. Zhang, C.L. Chen, G.X. Zhao, X. Yang, J. X. Li and X. K. Wang, “Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles”, ACS Appl. Mater. Inter. 4(9), 4991–5000 (2012). http://dx.doi.org/10.1021/am301358b
A. Roy and J. T. Bhattacharya, “Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes”, Chem. Eng. J. 211, 493–500 (2012). http://dx.doi.org/10.1016/j.cej.2012.09.097
D. K. Venkata Ramana, Jae Su Yu and K. Seshaiah, “Silver nanoparticles deposited multiwalled carbon nanotubes for removal of Cu(II) and Cd(II) from water: Surface, kinetic, equilibrium, and thermal adsorption properties”, Chem. Eng. J. 223, 806–815 (2013). http://dx.doi.org/10.1016/j.cej.2013.03.001
J. Gao, F. Q. Liu, P. P. Ling, J. T. Lei, L. J. Li, C. H. Li and A. M. Li, “High efficient removal of Cu(II) by a chelating resin from strong acidic solutions: Complex formation and DFT certification”, Chem. Eng. J. 222, 240–247 (2013). http://dx.doi.org/10.1016/j.cej.2013.02.055
K. G. Bhattacharyya and S. S. Gupta, “Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics”, Desalination 272(1–3), 66–75 (2011). http://dx.doi.org/10.1016/j.desal.2011.01.001
W. Q. Wu, Y. Yang, H. H. Zhou, T. T. Ye, Z. Y. Huang, R. Liu and Y. F. Kuang, “Highly efficient removal of Cu(II) from aqueous solution by using graphene oxide”, Water Air Soil. Poll. 224(1), 1372 (1–8) (2013). http://dx.doi.org/10.1007/s11270-012-1372-5
X. W. Wu, H. W. Ma, L. T. Zhang and F. J. Wang, “Adsorption properties and mechanism of mesoporous adsorbents prepared with fly ash for removal of Cu(II) in aqueous solution”, Appl. Surf. Sci. 261, 902–907 (2012). http://dx.doi.org/10.1016/j.apsusc.2012.08.122
D. Li, M. B. MüLler, S. Gilje, R. B. Kaner and G. G. Wallace, “Processable aqueous dispersions of graphene nanosheets”, Nat. Nanotechnol. 3(2), 101–105 (2008). http://dx.doi.org/10.1038/nnano.2007.451
L. H. Aia, C. Zhang and Z. Chen, “Removal of methylene blue from aqueous solution by asolvothermal synthesized graphene/magnetite composite”, J. Hazard. Mater. 192(3), 1515–1524 (2011). http://dx.doi.org/10.1016/j.jhazmat.2011.06.068
T. S. Sreeprasad, S. S. Gupt, S. M. Maliyekkal and T. Pradeep, “Immobilized graphene-based composite from asphalt: Facile synthesis and application in water purification”, J. Hazard. Mater. 246, 213–220 (2013). http://dx.doi.org/10.1016/j.jhazmat.2012.12.022
K. C. Kemp, H. Seema, M. Saleh and N. H. Le, “Environmental applications using graphene composites: water remediation and gas adsorption”. Nanoscale 5(8), 3149–3171 (2013). http://dx.doi.org/10.1039/c3nr33708a
V. Georgakilas, M. Otyepka and A. B. Bourlinos, “Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications”, Chem. Rev. 112(11), 6156–6214 (2012). http://dx.doi.org/10.1021/cr3000412
V. Chandra, J. Park and Y. Chun, “Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal”, ACS Nano 4(7), 3979–3986 (2010). http://dx.doi.org/10.1021/nn1008897
Z. H. Huang, X. Y. Heng, W. Lv, M. Wang, Q. H. Yang and F. Y. Kang, “Adsorption of Lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets”, Langmuir 27(12), 7558–7562 (2011). http://dx.doi.org/10.1021/la200606r
Y. W. Zhang, H. L. Ma, J. Peng, M. L. Zhai and Z. Z. Yu, “Cr(VI) removal from aqueous solution using chemically reduced and functionalized graphene oxide”, J. Mater. Sci. 48(5), 1883–1889 (2013). http://dx.doi.org/10.1007/s10853-012-6951-8
V. Chandra and K. S. Kim, “Highly selective adsorption of Hg2+ by a polypyrrole-reduced graphene oxide composite”, Chem. Commun. 47(13), 3942–3944 (2011). http://dx.doi.org/10.1039/c1cc00005e
K. Moon, J. H. Lee, R. S. Ruoff and H. Lee, “Reduced graphene oxide by chemical graphitization”, Nat. commun. 1, 73(1–6) (2010). http://dx.doi.org/10.1038/ncomms1067
Q. L Du, M. B. Zheng, L. F. Zhang, Y. W. Wang, J. H. Chen, L. P. Xue, W. J. Dai, G. B. Ji and J. M. Cao, “Preparation of functionalized graphene sheets by a low-temperature thermal exfoliation approach and their electrochemical supercapacitive behaviors”, Electrochim. Acta 55(12), 3897–3903 (2010). http://dx.doi.org/10.1016/j.electacta.2010.01.089
D. F. Han, C. S. Shan, L. P. Guo, L. Niu and D. X. Han, “Electro-oxidation of ascorbic acid on PVP-stabilized graphene electrode”, Chem. Res. Chinese U. 26(2), 287–290 (2010).
Q. F. Lu, J. Yu, J. Z. Gao, W. Yang and Y. Li, “Glowdischarge Electrolysis Plasma Induced Synthesis of Polyvinylpyrrolidone/Acrylic Acid Hydrogel and its Adsorption Properties for Heavy-metal Ions”, Plasma Process. Polym. 8(9), 803–814 (2011). http://dx.doi.org/10.1002/ppap.201000144
J. H. Zhao, W. Z. Yuan, A. H. Xu, F. Ai, Y. W. Lu and Y. M. Zhang, “Perfluorinated sulfonic acid ionomer/poly(N-vinylpyrrolidone) nanofiber membranes: Electrospinning fabrication, water stability, and metal ion removal applications”, React. Funct. Polym. 71(11), 1102–1109 (2011). http://dx.doi.org/10.1016/j.reactfunctpolym.2011.08.007
Y. H. Zhang, K. G. Zhou, K. F. Xie, H. L. Zhang, Y. Peng and C. W. Wang, “Tuning the magnetic and transport properties of metal adsorbed graphene by co-adsorption with 1,2-dichlorobenzene”, Phys. Chem. Chem. Phys 14(33), 11626–11632 (2012). http://dx.doi.org/10.1039/c2cp41370a