Polyetheramide Templated Synthesis of Monodisperse Mn3O4 Nanoparticles with Controlled Size and Study of the Electrochemical Properties
Corresponding Author: Xingmao Jiang
Nano-Micro Letters,
Vol. 6 No. 1 (2014), Article Number: 38-45
Abstract
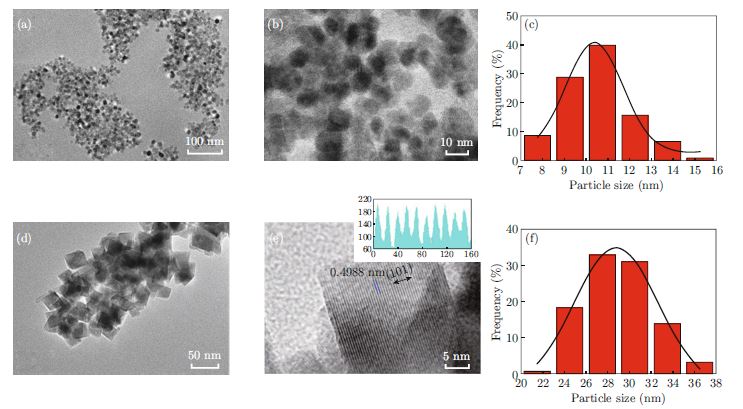
Monodisperse Mn3O4 nanoparticles were prepared solvothermally starting from manganese acetate by using polyether amide block copolymers (Pebax2533) as a template in isopropanol. The diameter of the nanoparticles in the range of 8.7 nm∼31.5 nm was decreased with increase of Pebax2533 concentration. The electrochemical properties and application in supercapacitor of Mn3O4 nanoparticles were further studied. The results showed that smaller nanoparticles had a larger capacitance. The higher capacitance of 217.5 F/g at a current density of 0.5 A/g was obtained on 8.7 nm Mn3O4 nanoparticles. The specific capacitance retention of 82% was maintained after 500 times of continuous charge-discharge cycles.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- B. E. Conway, “Electrochemical supercapacitors scientific fundamentals and technological applications”, New York: Plenum Publishers, 1999.
- (a)T. Mei, T. Li, H. Bi, L. Wang, Y. Zhu and Y. T. Qian, “Synthesis and Electrical Capacitance of Carbon Nanoplates”, Eur. J. Inorg. Chem. 2010(27), 4314–4320 (2010). http://dx.doi.org/10.1002/ejic.201000387; (b) B. P. Bastakoti, H. Oveisi, C. C. Hu, K. C. W. Wu, N. Suzuki, K. Takai, Y. Kamachi, M. Imura and Y. Yamauchi, “Mesoporous carbon incorporated with In2O3 nanoparticles as high-performance supercapacitors”, Eur. J. Inorg. Chem. 2013(7), 1109–1112 (2013). http://dx.doi.org/10.1002/ejic.201201311
- V. V. Pan’kov, “Interaction of (MnZn)O solid solutions with Fe2O3 as intermediate stage of formation of MnZn ferrites”, Ceram. Int. 14(2), 87–91 (1988). http://dx.doi.org/10.1016/S0272-8842(88)80003-8
- D. K. Kim, P. Muralidharan, H. W. Lee, R. Ruffo, Y. Yang, C. K. Chan, H. Peng, R. A. Huggins and Y. Cui, “Spinel LiMn2O4 nanorods as lithium ion battery cathodes”, Nano Lett. 8(11), 3948–3952 (2008). http://dx.doi.org/10.1021/nl8024328
- E. R. Stobbe, B. A. de Boer and J. W. Geus, “The reduction and oxidation behaviour of manganese oxides”, Catal. Today 47(1–4), 161–167 (1999). http://dx.doi.org/10.1016/S0920-5861(98)00296-X
- E. J. Grootendorst, Y. Verbeek and V. Ponec, “The role of the Mars and Van Krevelen mechanism in the selective oxidation of nitrosobenzene and the deoxygenation of nitrobenzene on oxidic catalysts”, J. Catal. 157(2), 706–712 (1995). http://dx.doi.org/10.1006/jcat.1995.1336
- W. S. Kijlstra, J. C. M. L. Daamen, J. M. van de Graaf, B. van der Linden, E. K. Poels and A. Bliek, “Inhibiting and deactivating effects of water on the selective catalytic reduction of nitric oxide with ammonia over MnOx/Al2O3”, Appl. Catal. B-Environ. 7(3–4), 337–357 (1996). http://dx.doi.org/10.1016/0926-3373(95)00052-6
- (a)Z. Chen, J. K. L. Lai and C. H. Shek, “Evolution of electronic structure and spectral evaluation in single-crystal Mn3O4 nanorods”, J. Chem. Phys. 124(18), 184707–184715 (2006). http://dx.doi.org/10.1063/1.2199848; (b) J. W. Lee, A. S. Hall, J. D. Kim and T. E. Mallouk, “A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability”, Chem. Mater. 24(6), 1158–1164 (2012). http://dx.doi.org/10.1021/cm203697w; (c) D. Wang, Y. Li, Q. Wang and T. Wang, “Facile synthesis of porous Mn3O4 nano-crystal-graphene nanocomposites for electrochemical supercapacitors”, Eur. J. Inorg. Chem. 2012(4), 628–635 (2012). http://dx.doi.org/10.1002/ejic.201100983
- Y. Q. Chang, X. Y. Xu, X. H. Luo, C. P. Chen and D. P. Yu, “Synthesis and characterization of Mn3O4 nanoparticles”, J. Cryst. Growth 264(1–3), 232–236 (2004). http://dx.doi.org/10.1016/j.jcrysgro.2003.11.11
- Y. Hu, J. Chen, X. Xue and T. Li, “Synthesis of monodispersed single-crystal compass-shaped Mn3O4 via gamma-ray irradiation”, Mater. Lett. 60(3), 383–385 (2006). http://dx.doi.org/10.1016/j.matlet.2005.08.056
- P. Z. Si, E. Brück, Z. D. Zhang, O. Tegus, W. S. Zhang, K. H. J. Buschow and J. C. P. Klaasse, “Structural and magnetic properties of Mn nanoparticles prepared by arc-discharge”, Mater. Res. Bull. 40(1), 29–37 (2005). http://dx.doi.org/10.1016/j.materresbull.2004.09.010
- W. X. Zhang, C. Wang, X. M. Zhang, Y. Xie and Y. T. Qian, “Low temperature synthesis of nanocrystalline Mn3O4 by a solvothermal method”, Solid State Ionics 117(3–4), 331–335 (1999). http://dx.doi.org/10.1016/S0167-2738(98)00432-9
- S. Ardizzone, C. L. Bianchi and D. Tirelli, “Mn3O4 and γ-MnOOH powders, preparation, phase composition and XPS characterisation”, Colloid. Surface. A 134(3), 305–312 (1998). http://dx.doi.org/10.1016/S0927-7757(97)00219-7
- (a)F. H. Schacher, P. A. Rupar and I. Manners, “Functional block copolymers: nanostructured materials with emerging applications”, Angew. Chem. Int. Edit. 51(32), 7898–7921 (2012). http://dx.doi.org/10.1002/anie.201200310; (b) Y. Mai and A. Eisenberg, “Selective localization of preformed nanoparticles in morphologically controllable block copolymer aggregates in solution”, Accounts Chem. Res. 45(10), 1657–1666 (2012). http://dx.doi.org/10.1021/ar2003144
- (a)Y. Li, H. Tan, X. X. Yang, B. Goris, J. Verbeeck, S. Bals, P. Colson, R. Cloots, G. Van Tendeloo and B. L. Su, “Well shaped Mn3O4 nano-octahedra with anomalous magnetic behavior and enhanced photodecomposition properties”, Small 7(4), 475–483 (2011). http://dx.doi.org/10.1002/smll.201001403; (b) W. Wang, T. Yang, G. Yan and H. Li, “Synthesis of Mn3O4 hollow octahedrons and their possible growth mechanism”, Mater. Lett. 82, 237–239 (2012). http://dx.doi.org/10.1016/j.matlet.2012.05.070; (c) K. An, M. Park, J. H. Yu, H. B. Na, N. Lee, J. Park, S. H. Choi, I. C. Song, W. K. Moon and T. Hyeon, “Synthesis of uniformly sized manganese oxide nanocrystals with various sizes and shapes and characterization of their T1 magnetic resonance relaxivity”, Eur. J. Inorg. Chem. 2012(12), 2148–2155 (2012). http://dx.doi.org/10.1002/ejic.201101193; (d) M. Liu and H. C. Zeng, “Spontaneous formations of superlattices and supracrystals from various forms of Mn3O4 nanocrystals”, Cryst. Growth Des. 12(11), 5561–5570 (2012). http://dx.doi.org/10.1021/cg3011103
- X. Ren, J. Ren, H. Li, S. Feng and M. Deng, “Poly (amide-6-b-ethylene oxide) multilayer composite membrane for carbon dioxide separation”, Int. J. Greenh. Gas Con. 8, 111–120 (2012). http://dx.doi.org/10.1016/j.ijggc.2012.01.017
- N. L. Le, Y. Wang and T. S. Chung, “Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation”, J. Membrane Sci. 379(1–2), 174–183 (2011). http://dx.doi.org/10.1016/j.memsci.2011.05.060
- E. Tocci, L. D. Lorenzo, A. Gugliuzza, M. Macchione and E. Drioli, “Pure and modified Co-poly(amide-12-b-ethylene oxide) membranes for gas separation studied by molecular investigations”, AIP Conf. Proc. 1298(1), 724–727 (2010). http://dx.doi.org/10.1063/1.3516418
- T. Kamal, S. Y. Park, J. H. Park and Y. W. Chang, “Structural evolution of poly(ether-b-amide12) elastomers during the uniaxial stretching: An in situ wide-angle X-ray scattering study”, Macromol. Res. 20(7), 725–731 (2012). http://dx.doi.org/10.1007/s13233-012-0109-z
- A. Moses Ezhil Raj, S. G. Victoria, V. B. Jothy, C. Ravidhas, J. Wollschläger, M. Suendorf, M. Neumann, M. Jayachandran and C. Sanjeeviraja, “XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique”, Appl. Surf. Sci., 256(9), 2920–2926 (2010). http://dx.doi.org/10.1016/j.apsusc.2009.11.051
- M. Oku, K. Hirokawa and S. Keda, “X-ray photoelectron spectroscopy of manganese—oxygen systems”, J. Electron Spectrosc. 7(5), 465–473 (1975). http://dx.doi.org/10.1016/0368-2048(75)85010-9
- J. S. Foord, R. B. Jackman and G. C. Allen, “An X-ray photoelectron spectroscopic investigation of the oxidation of manganese”, Philos. Mag. A 49(5), 657–663 (1984). http://dx.doi.org/10.1080/01418618408233293
- Y. Fan, X. Zhang, Y. Liu, Q. Cai and J. Zhang, “One-pot hydrothermal synthesis of Mn3O4/graphene nanocomposite for supercapacitors”, Mater. Lett. 95, 153–156 (2013). http://dx.doi.org/10.1016/j.matlet.2012.12.110
- X. M. Jiang and C. J. Brinker, “Rigid templating of high surface-area, mesoporous, nanocrystalline rutile using a polyether block amide copolymer template”, Chem. Commun. 46(33), 6123–6125 (2010). http://dx.doi.org/10.1039/C0CC01394C
- L. X. Zhu, S. Zhang, Y. H. Cui, H. H. Song and X. H. Chen, “One step synthesis and capacitive performance of grapheme nanosheets/Mn3O4 composite”, Electrochim. Acta 89, 18–23 (2013). http://dx.doi.org/10.1016/j.electacta.2012.10.157
- Michio Inagaki, Hidetaka Konno and Osamu Tanaike, “Carbon materials for electrochemical capacitors”, J. Power Sources 195(24), 7880–7903 (2010). http://dx.doi.org/10.1016/j.jpowsour.2010.06.036
- S. Nagamuthu, S. Vijayakumar and G. Muralidharan, “Synthesis of Mn3O4/Amorphous carbon nanoparticles as electrode material for high performance supercapacitor applications”, Energ. Fuel. 27(6), 3508–3515 (2013). http://dx.doi.org/10.1021/ef400212b
- D. P. Dubal and R. Holze, “Self-assembly of stacked layers of Mn3O4 nanosheets using a scalable chemical strategy for enhanced, flexible, electrochemical energy storage”, J. Power Sources 238, 274–282 (2013). http://dx.doi.org/10.1016/j.jpowsour.2013.01.198
- Y. Z. Wu, S. Q. Liu, H. Y. Wang, X. W. Wang, X. Zhang and G. H. Jin. “A novel solvothermal synthesis of Mn3O4/grapheme composites for supercapacitors”, Electrochim. Acta 90, 210–218 (2013). http://dx.doi.org/10.1016/j.electacta.2012.11.124
- K. Sankar, D. Kalpana and R. Selvan, “Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications”, J. Appl. Electrochem. 42(7), 463–470 (2012). http://dx.doi.org/10.1007/s10800-012-0424-2
References
B. E. Conway, “Electrochemical supercapacitors scientific fundamentals and technological applications”, New York: Plenum Publishers, 1999.
(a)T. Mei, T. Li, H. Bi, L. Wang, Y. Zhu and Y. T. Qian, “Synthesis and Electrical Capacitance of Carbon Nanoplates”, Eur. J. Inorg. Chem. 2010(27), 4314–4320 (2010). http://dx.doi.org/10.1002/ejic.201000387; (b) B. P. Bastakoti, H. Oveisi, C. C. Hu, K. C. W. Wu, N. Suzuki, K. Takai, Y. Kamachi, M. Imura and Y. Yamauchi, “Mesoporous carbon incorporated with In2O3 nanoparticles as high-performance supercapacitors”, Eur. J. Inorg. Chem. 2013(7), 1109–1112 (2013). http://dx.doi.org/10.1002/ejic.201201311
V. V. Pan’kov, “Interaction of (MnZn)O solid solutions with Fe2O3 as intermediate stage of formation of MnZn ferrites”, Ceram. Int. 14(2), 87–91 (1988). http://dx.doi.org/10.1016/S0272-8842(88)80003-8
D. K. Kim, P. Muralidharan, H. W. Lee, R. Ruffo, Y. Yang, C. K. Chan, H. Peng, R. A. Huggins and Y. Cui, “Spinel LiMn2O4 nanorods as lithium ion battery cathodes”, Nano Lett. 8(11), 3948–3952 (2008). http://dx.doi.org/10.1021/nl8024328
E. R. Stobbe, B. A. de Boer and J. W. Geus, “The reduction and oxidation behaviour of manganese oxides”, Catal. Today 47(1–4), 161–167 (1999). http://dx.doi.org/10.1016/S0920-5861(98)00296-X
E. J. Grootendorst, Y. Verbeek and V. Ponec, “The role of the Mars and Van Krevelen mechanism in the selective oxidation of nitrosobenzene and the deoxygenation of nitrobenzene on oxidic catalysts”, J. Catal. 157(2), 706–712 (1995). http://dx.doi.org/10.1006/jcat.1995.1336
W. S. Kijlstra, J. C. M. L. Daamen, J. M. van de Graaf, B. van der Linden, E. K. Poels and A. Bliek, “Inhibiting and deactivating effects of water on the selective catalytic reduction of nitric oxide with ammonia over MnOx/Al2O3”, Appl. Catal. B-Environ. 7(3–4), 337–357 (1996). http://dx.doi.org/10.1016/0926-3373(95)00052-6
(a)Z. Chen, J. K. L. Lai and C. H. Shek, “Evolution of electronic structure and spectral evaluation in single-crystal Mn3O4 nanorods”, J. Chem. Phys. 124(18), 184707–184715 (2006). http://dx.doi.org/10.1063/1.2199848; (b) J. W. Lee, A. S. Hall, J. D. Kim and T. E. Mallouk, “A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability”, Chem. Mater. 24(6), 1158–1164 (2012). http://dx.doi.org/10.1021/cm203697w; (c) D. Wang, Y. Li, Q. Wang and T. Wang, “Facile synthesis of porous Mn3O4 nano-crystal-graphene nanocomposites for electrochemical supercapacitors”, Eur. J. Inorg. Chem. 2012(4), 628–635 (2012). http://dx.doi.org/10.1002/ejic.201100983
Y. Q. Chang, X. Y. Xu, X. H. Luo, C. P. Chen and D. P. Yu, “Synthesis and characterization of Mn3O4 nanoparticles”, J. Cryst. Growth 264(1–3), 232–236 (2004). http://dx.doi.org/10.1016/j.jcrysgro.2003.11.11
Y. Hu, J. Chen, X. Xue and T. Li, “Synthesis of monodispersed single-crystal compass-shaped Mn3O4 via gamma-ray irradiation”, Mater. Lett. 60(3), 383–385 (2006). http://dx.doi.org/10.1016/j.matlet.2005.08.056
P. Z. Si, E. Brück, Z. D. Zhang, O. Tegus, W. S. Zhang, K. H. J. Buschow and J. C. P. Klaasse, “Structural and magnetic properties of Mn nanoparticles prepared by arc-discharge”, Mater. Res. Bull. 40(1), 29–37 (2005). http://dx.doi.org/10.1016/j.materresbull.2004.09.010
W. X. Zhang, C. Wang, X. M. Zhang, Y. Xie and Y. T. Qian, “Low temperature synthesis of nanocrystalline Mn3O4 by a solvothermal method”, Solid State Ionics 117(3–4), 331–335 (1999). http://dx.doi.org/10.1016/S0167-2738(98)00432-9
S. Ardizzone, C. L. Bianchi and D. Tirelli, “Mn3O4 and γ-MnOOH powders, preparation, phase composition and XPS characterisation”, Colloid. Surface. A 134(3), 305–312 (1998). http://dx.doi.org/10.1016/S0927-7757(97)00219-7
(a)F. H. Schacher, P. A. Rupar and I. Manners, “Functional block copolymers: nanostructured materials with emerging applications”, Angew. Chem. Int. Edit. 51(32), 7898–7921 (2012). http://dx.doi.org/10.1002/anie.201200310; (b) Y. Mai and A. Eisenberg, “Selective localization of preformed nanoparticles in morphologically controllable block copolymer aggregates in solution”, Accounts Chem. Res. 45(10), 1657–1666 (2012). http://dx.doi.org/10.1021/ar2003144
(a)Y. Li, H. Tan, X. X. Yang, B. Goris, J. Verbeeck, S. Bals, P. Colson, R. Cloots, G. Van Tendeloo and B. L. Su, “Well shaped Mn3O4 nano-octahedra with anomalous magnetic behavior and enhanced photodecomposition properties”, Small 7(4), 475–483 (2011). http://dx.doi.org/10.1002/smll.201001403; (b) W. Wang, T. Yang, G. Yan and H. Li, “Synthesis of Mn3O4 hollow octahedrons and their possible growth mechanism”, Mater. Lett. 82, 237–239 (2012). http://dx.doi.org/10.1016/j.matlet.2012.05.070; (c) K. An, M. Park, J. H. Yu, H. B. Na, N. Lee, J. Park, S. H. Choi, I. C. Song, W. K. Moon and T. Hyeon, “Synthesis of uniformly sized manganese oxide nanocrystals with various sizes and shapes and characterization of their T1 magnetic resonance relaxivity”, Eur. J. Inorg. Chem. 2012(12), 2148–2155 (2012). http://dx.doi.org/10.1002/ejic.201101193; (d) M. Liu and H. C. Zeng, “Spontaneous formations of superlattices and supracrystals from various forms of Mn3O4 nanocrystals”, Cryst. Growth Des. 12(11), 5561–5570 (2012). http://dx.doi.org/10.1021/cg3011103
X. Ren, J. Ren, H. Li, S. Feng and M. Deng, “Poly (amide-6-b-ethylene oxide) multilayer composite membrane for carbon dioxide separation”, Int. J. Greenh. Gas Con. 8, 111–120 (2012). http://dx.doi.org/10.1016/j.ijggc.2012.01.017
N. L. Le, Y. Wang and T. S. Chung, “Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation”, J. Membrane Sci. 379(1–2), 174–183 (2011). http://dx.doi.org/10.1016/j.memsci.2011.05.060
E. Tocci, L. D. Lorenzo, A. Gugliuzza, M. Macchione and E. Drioli, “Pure and modified Co-poly(amide-12-b-ethylene oxide) membranes for gas separation studied by molecular investigations”, AIP Conf. Proc. 1298(1), 724–727 (2010). http://dx.doi.org/10.1063/1.3516418
T. Kamal, S. Y. Park, J. H. Park and Y. W. Chang, “Structural evolution of poly(ether-b-amide12) elastomers during the uniaxial stretching: An in situ wide-angle X-ray scattering study”, Macromol. Res. 20(7), 725–731 (2012). http://dx.doi.org/10.1007/s13233-012-0109-z
A. Moses Ezhil Raj, S. G. Victoria, V. B. Jothy, C. Ravidhas, J. Wollschläger, M. Suendorf, M. Neumann, M. Jayachandran and C. Sanjeeviraja, “XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique”, Appl. Surf. Sci., 256(9), 2920–2926 (2010). http://dx.doi.org/10.1016/j.apsusc.2009.11.051
M. Oku, K. Hirokawa and S. Keda, “X-ray photoelectron spectroscopy of manganese—oxygen systems”, J. Electron Spectrosc. 7(5), 465–473 (1975). http://dx.doi.org/10.1016/0368-2048(75)85010-9
J. S. Foord, R. B. Jackman and G. C. Allen, “An X-ray photoelectron spectroscopic investigation of the oxidation of manganese”, Philos. Mag. A 49(5), 657–663 (1984). http://dx.doi.org/10.1080/01418618408233293
Y. Fan, X. Zhang, Y. Liu, Q. Cai and J. Zhang, “One-pot hydrothermal synthesis of Mn3O4/graphene nanocomposite for supercapacitors”, Mater. Lett. 95, 153–156 (2013). http://dx.doi.org/10.1016/j.matlet.2012.12.110
X. M. Jiang and C. J. Brinker, “Rigid templating of high surface-area, mesoporous, nanocrystalline rutile using a polyether block amide copolymer template”, Chem. Commun. 46(33), 6123–6125 (2010). http://dx.doi.org/10.1039/C0CC01394C
L. X. Zhu, S. Zhang, Y. H. Cui, H. H. Song and X. H. Chen, “One step synthesis and capacitive performance of grapheme nanosheets/Mn3O4 composite”, Electrochim. Acta 89, 18–23 (2013). http://dx.doi.org/10.1016/j.electacta.2012.10.157
Michio Inagaki, Hidetaka Konno and Osamu Tanaike, “Carbon materials for electrochemical capacitors”, J. Power Sources 195(24), 7880–7903 (2010). http://dx.doi.org/10.1016/j.jpowsour.2010.06.036
S. Nagamuthu, S. Vijayakumar and G. Muralidharan, “Synthesis of Mn3O4/Amorphous carbon nanoparticles as electrode material for high performance supercapacitor applications”, Energ. Fuel. 27(6), 3508–3515 (2013). http://dx.doi.org/10.1021/ef400212b
D. P. Dubal and R. Holze, “Self-assembly of stacked layers of Mn3O4 nanosheets using a scalable chemical strategy for enhanced, flexible, electrochemical energy storage”, J. Power Sources 238, 274–282 (2013). http://dx.doi.org/10.1016/j.jpowsour.2013.01.198
Y. Z. Wu, S. Q. Liu, H. Y. Wang, X. W. Wang, X. Zhang and G. H. Jin. “A novel solvothermal synthesis of Mn3O4/grapheme composites for supercapacitors”, Electrochim. Acta 90, 210–218 (2013). http://dx.doi.org/10.1016/j.electacta.2012.11.124
K. Sankar, D. Kalpana and R. Selvan, “Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications”, J. Appl. Electrochem. 42(7), 463–470 (2012). http://dx.doi.org/10.1007/s10800-012-0424-2