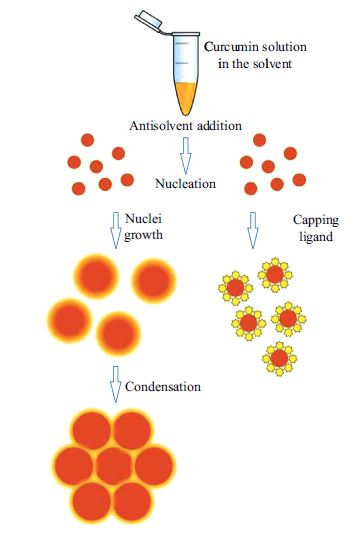
High-Throughput Screening of Nanoparticle-Stabilizing Ligands: Application to Preparing Antimicrobial Curcumin Nanoparticles by Antisolvent Precipitation
Corresponding Author: Victor Rodov
Nano-Micro Letters,
Vol. 7 No. 1 (2015), Article Number: 68-79
Abstract
Water-dispersible curcumin nanoparticles were prepared by bottom-up antisolvent precipitation approach. A new high-throughput screening technique was developed for selecting appropriate ligands stabilizing the nanoparticles in aqueous medium and improving their performance. The initial set of twenty-eight potential stabilizing ligands was evaluated based on their capacity to improve curcumin dispersibility in aqueous medium. The performance of four promising ligands (amino acid proline, polyphenol tannic acid, polycation Polyquaternium 10, and neutral polymer polyvinylpyrrolidone) was tested in ultrasound-aided antisolvent precipitation trials. Using the selected stabilizing ligands diminished the average particle size from ca. 1,200 to 170–230 nm, reduced their dispersity, improved stability, and allowed reaching curcumin concentration of up to 1.4 mM in aqueous medium. Storage stability of the aqueous nanodispersions varied from 2 days to 2 weeks, depending on stabilizing ligand. Studying the effects of ionic strength and pH on size and ζ-potential of the particles suggested that electrostatic forces and hydrophobic interactions could be the major factors affecting their stability. The ligand-protected nanoparticles showed minimal inhibitory concentration of 400 or 500 µM toward Escherichia coli. We suggest that the presented screening approach may be useful for preparing nanoparticles of various poorly water-soluble bioactive materials.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- European Food Safety Authority (EFSA), Panel on food additives and nutrient sources added to food, scientific opinion on the re-evaluation of curcumin (E 100) as a food additive. EFSA J. 8(9), 1679–1725 (2010). doi:10.2903/j.efsa.2010.1679http://www.efsa.europa.eu/en/efsajournal/pub/1679.htm. Accessed 25 October 2014
- E. Schraufstätter, H. Bernt, Antibacterial action of curcumin and related compounds. Nature 164(4167), 456–457 (1949). doi:10.1038/164456a0
- Y. Wang, Z. Lu, H. Wu, F. Lv, Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 136(1), 71–74 (2009). doi:10.1016/j.ijfoodmicro.2009.09.001
- R. De, P. Kundu, S. Swarnakar, T. Ramamurthy, A. Chowdhury, G.B. Nair, A.K. Mukhopadhyay, Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 53(4), 1592–1597 (2009). doi:10.1128/AAC.01242-08
- C.V.B. Martins, D.L. Da Silva, A.T.M. Neres, T.F.F. Magalhaes, G.A. Watanabe, L.V. Modolo, A.A. Sabino, A. De Fátima, M.A. De Resende, Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 63(2), 337–339 (2009). doi:10.1093/jac/dkn488
- A. Mobasheri, Y. Henrotin, H.K. Biesalski, M. Shakibaei, Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int. J. Mol. Sci. 13(4), 4202–4232 (2012). doi:10.3390/ijms13044202
- V. Rodov, B. Horev, Y. Vinokur, D. Beno-Mualem, R. Choudhary, S. Makwana, J. Haddock, N. Dogra, P. Kohli, S. Droby, Antimicrobial and antioxidant activity of phenylpropanoids encapsulated in methylated β-cyclodextrin and in polydiacetylene nanovesicles, in 5th Int. Symp. on Human Health Effects of Fruits and Vegetables (FAV Health 2012), Dharwad, India, 7 Jan 2013, p. 18
- C.M. Keck, R.H. Muller, Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 62(1), 3–16 (2006). doi:10.1016/j.ejpb.2005.05.009
- H.S. Ali, P. York, A. Ali, N. Blagden, Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J. Control Release 149(2), 175–181 (2011). doi:10.1016/j.jconrel.2010.10.007
- Z. Zheng, X. Zhang, D. Carbo, C. Clark, C.A. Nathan, Y. Lvov, Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir 26(11), 7679–7681 (2010). doi:10.1021/la101246a
- R.K. Bhawana, H.S. Basniwal, V.K. Buttar, N. Jain, Jain, Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 59(5), 2056–2061 (2011). doi:10.1021/jf104402t
- M. Kakran, N.G. Sahoo, I.L. Tan, L. Li, Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanopart. Res. 14(3), 1–11 (2012). doi:10.1007/s11051-012-0757-0
- X. Meng, Y. Chen, S.R. Chowdhury, D. Yang, S. Mitra, Stabilizing dispersions of hydrophobic drug molecules using cellulose ethers during anti-solvent synthesis of micro-particulates. Colloid Surf. B 70(1), 7–14 (2009). doi:10.1016/j.colsurfb.2008.12.002
- S.L. Raghavan, K. Schuessel, A. Davis, J. Hadgraft, Formation and stabilisation of triclosan colloidal suspensions using supersaturated systems. Int. J. Pharm. 261(1), 153–158 (2003). doi:10.1016/S0378-5173(03)00299-0
- B.E. Rabinow, Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 3(9), 785–796 (2004). doi:10.1038/nrd1494
- J.Y. Choi, J.Y. Yoo, H.S. Kwak, B.U. Nam, J. Lee, Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 5(5), 472–474 (2005). doi:10.1016/j.cap.2005.01.012
- T.O. McDonald, L.M. Tatham, F.Y. Southworth, M. Giardiello, P. Martin, N.J. Liptrott, A. Owen, S.R. Rannard, High-throughput nanoprecipitation of the organic antimicrobial triclosan and enhancement of activity against Escherichia coli. J. Mater. Chem. B 1(35), 4455–4465 (2013). doi:10.1039/c3tb20543f
- D. Rautaray, S. Mandal, M. Sastry, Synthesis of hydroxyapatite crystals using amino acid-capped gold nanoparticles as a scaffold. Langmuir 21(11), 5185–5191 (2005). doi:10.1021/la048541f
- R. Gonzalez-McQuire, J.Y. Chane-Ching, E. Vignaud, A. Lebugle, S. Mann, Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 14(14), 2277–2281 (2004). doi:10.1039/b400317a
- J.A. Ho, H.C. Chang, W.T. Su, DOPA-mediated reduction allows the facile synthesis of fluorescent gold nanoclusters for use as sensing probes for ferric ions. Anal. Chem. 84(7), 3246–3253 (2012). doi:10.1021/ac203362g
- D. van Lierop, Ž. Krpetić, L. Guerrini, I.A. Larmour, J.A. Dougan, K. Faulds, D. Graham, Positively charged silver nanoparticles and their effect on surface-enhanced Raman scattering of dye-labelled oligonucleotides. Chem. Commun. 48(66), 8192–8194 (2012). doi:10.1039/c2cc31731a
- S.A. Moreno-Álvarez, G.A. Martínez-Castañón, N. Niño-Martínez, J.F. Reyes-Macías, N. Patiño-Marín, J.P. Loyola-Rodríguez, F. Ruiz, Preparation and bactericide activity of gallic acid stabilized gold nanoparticles. J. Nanopart. Res. 12(8), 2741–2746 (2010). doi:10.1007/s11051-010-0060-x
- S.K. Nune, N. Chanda, R. Shukla, K. Katti, R.R. Kulkarni, S. Thilakavathy, S. Mekapothula, R. Kannan, K.V. Katti, Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 19(19), 2912–2920 (2009). doi:10.1039/b822015h
- S.A. Aromal, D. Philip, Facile one-pot synthesis of gold nanoparticles using tannic acid and its application in catalysis. Phys. E 44(7–8), 1692–1696 (2012). doi:10.1016/j.physe.2012.04.022
- M. Stobiecka, M. Hepel, Double-shell gold nanoparticle-based DNA-carriers with poly-l-lysine binding surface. Biomaterials 32(12), 3312–3321 (2011). doi:10.1016/j.biomaterials.2010.12.064
- R.K. Gangwar, V.A. Dhumale, D. Kumari, U.T. Nakate, S.W. Gosavi, R.B. Sharma, S.N. Kale, S. Datar, Conjugation of curcumin with PVP capped gold nanoparticles for improving bioavailability. Mater. Sci. Eng. C 32(8), 2659–2663 (2012). doi:10.1016/j.msec.2012.07.022
- H. Zhang, Y. Yang, W. Dai, D. Yang, S. Lu, Y. Ji, An aqueous-phase catalytic process for the selective hydrogenation of acetylene with monodisperse water soluble palladium nanoparticles as catalyst. Catal. Sci. Technol. 2(7), 1319–1323 (2012). doi:10.1039/c2cy20179h
- H. Huang, X. Yang, Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr. Res. 339(15), 2627–2631 (2004). doi:10.1016/j.carres.2004.08.005
- H.L. Ma, X.R. Qi, Y. Maitani, T. Nagai, Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 333(1), 177–186 (2007). doi:10.1016/j.ijpharm.2006.10.006
- I. Ghosh, S. Bose, R. Vippagunta, F. Harmon, Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 409(1), 260–268 (2011). doi:10.1016/j.ijpharm.2011.02.051
- R.J. Hunter, R.H. Ottewill, R.L. Rowell, Charge and potential distribution at interfaces, in Zeta potential in colloid science: principles and applications (Academic Press, London, 1981), pp. 11–58. doi: 10.1016/B978-0-12-361961-7.50006-7
- A.R. Patel, J. Seijen ten Hoorn, K.P. Velikov, Colloidal complexes from associated water soluble cellulose derivative (methylcellulose) and green tea polyphenol (epigallocatechin gallate). J. Colloid Interface Sci. 364(2), 317–323 (2011). doi:10.1016/j.jcis.2011.08.054
- J. Scheutjens, G.J. Fleer, Statistical-theory of the adsorption of interacting chain molecules. 2. Train, loop, and tail size distribution. J. Phys. Chem. 84(2), 178–190 (1980). doi:10.1021/j100439a011
- B. Derjaguin, L. Landau, Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged-particles in solutions of electrolytes. Prog. Surf. Sci. 43(1–4), 30–59 (1993). doi:10.1016/0079-6816(93)90013-L
- E.J.W. Verwey, Theory of the stability of lyophobic colloids. J. Phys. Colloid Chem. 51(3), 631–636 (1947). doi:10.1021/j150453a001
- B.P. Singh, R. Menchavez, C. Takai, M. Fuji, M. Takahashi, Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 291(1), 181–186 (2005). doi:10.1016/j.jcis.2005.04.091
References
European Food Safety Authority (EFSA), Panel on food additives and nutrient sources added to food, scientific opinion on the re-evaluation of curcumin (E 100) as a food additive. EFSA J. 8(9), 1679–1725 (2010). doi:10.2903/j.efsa.2010.1679http://www.efsa.europa.eu/en/efsajournal/pub/1679.htm. Accessed 25 October 2014
E. Schraufstätter, H. Bernt, Antibacterial action of curcumin and related compounds. Nature 164(4167), 456–457 (1949). doi:10.1038/164456a0
Y. Wang, Z. Lu, H. Wu, F. Lv, Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 136(1), 71–74 (2009). doi:10.1016/j.ijfoodmicro.2009.09.001
R. De, P. Kundu, S. Swarnakar, T. Ramamurthy, A. Chowdhury, G.B. Nair, A.K. Mukhopadhyay, Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 53(4), 1592–1597 (2009). doi:10.1128/AAC.01242-08
C.V.B. Martins, D.L. Da Silva, A.T.M. Neres, T.F.F. Magalhaes, G.A. Watanabe, L.V. Modolo, A.A. Sabino, A. De Fátima, M.A. De Resende, Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 63(2), 337–339 (2009). doi:10.1093/jac/dkn488
A. Mobasheri, Y. Henrotin, H.K. Biesalski, M. Shakibaei, Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int. J. Mol. Sci. 13(4), 4202–4232 (2012). doi:10.3390/ijms13044202
V. Rodov, B. Horev, Y. Vinokur, D. Beno-Mualem, R. Choudhary, S. Makwana, J. Haddock, N. Dogra, P. Kohli, S. Droby, Antimicrobial and antioxidant activity of phenylpropanoids encapsulated in methylated β-cyclodextrin and in polydiacetylene nanovesicles, in 5th Int. Symp. on Human Health Effects of Fruits and Vegetables (FAV Health 2012), Dharwad, India, 7 Jan 2013, p. 18
C.M. Keck, R.H. Muller, Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 62(1), 3–16 (2006). doi:10.1016/j.ejpb.2005.05.009
H.S. Ali, P. York, A. Ali, N. Blagden, Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J. Control Release 149(2), 175–181 (2011). doi:10.1016/j.jconrel.2010.10.007
Z. Zheng, X. Zhang, D. Carbo, C. Clark, C.A. Nathan, Y. Lvov, Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir 26(11), 7679–7681 (2010). doi:10.1021/la101246a
R.K. Bhawana, H.S. Basniwal, V.K. Buttar, N. Jain, Jain, Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 59(5), 2056–2061 (2011). doi:10.1021/jf104402t
M. Kakran, N.G. Sahoo, I.L. Tan, L. Li, Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanopart. Res. 14(3), 1–11 (2012). doi:10.1007/s11051-012-0757-0
X. Meng, Y. Chen, S.R. Chowdhury, D. Yang, S. Mitra, Stabilizing dispersions of hydrophobic drug molecules using cellulose ethers during anti-solvent synthesis of micro-particulates. Colloid Surf. B 70(1), 7–14 (2009). doi:10.1016/j.colsurfb.2008.12.002
S.L. Raghavan, K. Schuessel, A. Davis, J. Hadgraft, Formation and stabilisation of triclosan colloidal suspensions using supersaturated systems. Int. J. Pharm. 261(1), 153–158 (2003). doi:10.1016/S0378-5173(03)00299-0
B.E. Rabinow, Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 3(9), 785–796 (2004). doi:10.1038/nrd1494
J.Y. Choi, J.Y. Yoo, H.S. Kwak, B.U. Nam, J. Lee, Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 5(5), 472–474 (2005). doi:10.1016/j.cap.2005.01.012
T.O. McDonald, L.M. Tatham, F.Y. Southworth, M. Giardiello, P. Martin, N.J. Liptrott, A. Owen, S.R. Rannard, High-throughput nanoprecipitation of the organic antimicrobial triclosan and enhancement of activity against Escherichia coli. J. Mater. Chem. B 1(35), 4455–4465 (2013). doi:10.1039/c3tb20543f
D. Rautaray, S. Mandal, M. Sastry, Synthesis of hydroxyapatite crystals using amino acid-capped gold nanoparticles as a scaffold. Langmuir 21(11), 5185–5191 (2005). doi:10.1021/la048541f
R. Gonzalez-McQuire, J.Y. Chane-Ching, E. Vignaud, A. Lebugle, S. Mann, Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 14(14), 2277–2281 (2004). doi:10.1039/b400317a
J.A. Ho, H.C. Chang, W.T. Su, DOPA-mediated reduction allows the facile synthesis of fluorescent gold nanoclusters for use as sensing probes for ferric ions. Anal. Chem. 84(7), 3246–3253 (2012). doi:10.1021/ac203362g
D. van Lierop, Ž. Krpetić, L. Guerrini, I.A. Larmour, J.A. Dougan, K. Faulds, D. Graham, Positively charged silver nanoparticles and their effect on surface-enhanced Raman scattering of dye-labelled oligonucleotides. Chem. Commun. 48(66), 8192–8194 (2012). doi:10.1039/c2cc31731a
S.A. Moreno-Álvarez, G.A. Martínez-Castañón, N. Niño-Martínez, J.F. Reyes-Macías, N. Patiño-Marín, J.P. Loyola-Rodríguez, F. Ruiz, Preparation and bactericide activity of gallic acid stabilized gold nanoparticles. J. Nanopart. Res. 12(8), 2741–2746 (2010). doi:10.1007/s11051-010-0060-x
S.K. Nune, N. Chanda, R. Shukla, K. Katti, R.R. Kulkarni, S. Thilakavathy, S. Mekapothula, R. Kannan, K.V. Katti, Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 19(19), 2912–2920 (2009). doi:10.1039/b822015h
S.A. Aromal, D. Philip, Facile one-pot synthesis of gold nanoparticles using tannic acid and its application in catalysis. Phys. E 44(7–8), 1692–1696 (2012). doi:10.1016/j.physe.2012.04.022
M. Stobiecka, M. Hepel, Double-shell gold nanoparticle-based DNA-carriers with poly-l-lysine binding surface. Biomaterials 32(12), 3312–3321 (2011). doi:10.1016/j.biomaterials.2010.12.064
R.K. Gangwar, V.A. Dhumale, D. Kumari, U.T. Nakate, S.W. Gosavi, R.B. Sharma, S.N. Kale, S. Datar, Conjugation of curcumin with PVP capped gold nanoparticles for improving bioavailability. Mater. Sci. Eng. C 32(8), 2659–2663 (2012). doi:10.1016/j.msec.2012.07.022
H. Zhang, Y. Yang, W. Dai, D. Yang, S. Lu, Y. Ji, An aqueous-phase catalytic process for the selective hydrogenation of acetylene with monodisperse water soluble palladium nanoparticles as catalyst. Catal. Sci. Technol. 2(7), 1319–1323 (2012). doi:10.1039/c2cy20179h
H. Huang, X. Yang, Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr. Res. 339(15), 2627–2631 (2004). doi:10.1016/j.carres.2004.08.005
H.L. Ma, X.R. Qi, Y. Maitani, T. Nagai, Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 333(1), 177–186 (2007). doi:10.1016/j.ijpharm.2006.10.006
I. Ghosh, S. Bose, R. Vippagunta, F. Harmon, Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 409(1), 260–268 (2011). doi:10.1016/j.ijpharm.2011.02.051
R.J. Hunter, R.H. Ottewill, R.L. Rowell, Charge and potential distribution at interfaces, in Zeta potential in colloid science: principles and applications (Academic Press, London, 1981), pp. 11–58. doi: 10.1016/B978-0-12-361961-7.50006-7
A.R. Patel, J. Seijen ten Hoorn, K.P. Velikov, Colloidal complexes from associated water soluble cellulose derivative (methylcellulose) and green tea polyphenol (epigallocatechin gallate). J. Colloid Interface Sci. 364(2), 317–323 (2011). doi:10.1016/j.jcis.2011.08.054
J. Scheutjens, G.J. Fleer, Statistical-theory of the adsorption of interacting chain molecules. 2. Train, loop, and tail size distribution. J. Phys. Chem. 84(2), 178–190 (1980). doi:10.1021/j100439a011
B. Derjaguin, L. Landau, Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged-particles in solutions of electrolytes. Prog. Surf. Sci. 43(1–4), 30–59 (1993). doi:10.1016/0079-6816(93)90013-L
E.J.W. Verwey, Theory of the stability of lyophobic colloids. J. Phys. Colloid Chem. 51(3), 631–636 (1947). doi:10.1021/j150453a001
B.P. Singh, R. Menchavez, C. Takai, M. Fuji, M. Takahashi, Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 291(1), 181–186 (2005). doi:10.1016/j.jcis.2005.04.091