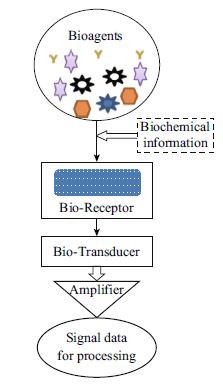
Biomedical Perspective of Electrochemical Nanobiosensor
Corresponding Author: Sunil Kumar Singh
Nano-Micro Letters,
Vol. 8 No. 3 (2016), Article Number: 193-203
Abstract
Electrochemical biosensor holds great promise in the biomedical area due to its enhanced specificity, sensitivity, label-free nature and cost effectiveness for rapid point-of-care detection of diseases at bedside. In this review, we are focusing on the working principle of electrochemical biosensor and how it can be employed in detecting biomarkers of fatal diseases like cancer, AIDS, hepatitis and cardiovascular diseases. Recent advances in the development of implantable biosensors and exploration of nanomaterials in fabrication of electrodes with increasing the sensitivity of biosensor for quick and easy detection of biomolecules have been elucidated in detail. Electrochemical-based detection of heavy metal ions which cause harmful effect on human health has been discussed. Key challenges associated with the electrochemical sensor and its future perspectives are also addressed.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- A. Hulanicki, S. Glab, F.O.L.K.E. Ingman, Chemical sensors: definitions and classification. Pure Appl. Chem. 63(9), 1247–1250 (1991). doi:10.1351/pac199163091247
- S.K. Arya, S. Saha, J.E. Ramirez-Vick, V. Gupta, S. Bhansali, S.P. Singh, Recent advances in ZnO nanostructures and thin films for biosensor applications: review. Anal. Chim. Acta 737, 1–21 (2012). doi:10.1016/j.aca.2012.05.048
- J. Wang, Sol–gel materials for electrochemical biosensors. Anal. Chim. Acta 399(1), 21–27 (1999). doi:10.1016/S0003-2670(99)00572-3
- J. Wang, D. Xu, A.N. Kawde, R. Polsky, Metal nanoparticle-based electrochemical stripping potentiometric detection of DNA hybridization. Anal. Chem. 73(22), 5576–5581 (2001). doi:10.1021/ac0107148
- S. Zhang, N. Wang, H. Yu, Y. Niu, C. Sun, Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 67(1), 15–22 (2005). doi:10.1016/j.bioelechem.2004.12.002
- J. Wang, Carbon–nanotube based electrochemical biosensors: a review. Electroanalysis 17(1), 7–14 (2005). doi:10.1002/elan.200403113
- J. Wang, Nanomaterial-based electrochemical biosensors. Analyst 130(4), 421–426 (2005). doi:10.1039/b414248a
- A. Zhu, Q. Qu, X. Shao, B. Kong, Y. Tian, Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew. Chem. 124(29), 7297–7301 (2012). doi:10.1002/ange.201109089
- K.J. Cash, H.A. Clark, Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 16(12), 584–593 (2010). doi:10.1016/j.molmed.2010.08.002
- S. Solé, A. Merkoci, S. Alegret, New materials for electrochemical sensing III beads. TrAC-Trend Anal. Chem. 20(2), 102–110 (2001). doi:10.1016/s0165-9936(00)00059-5
- H. Wang, Y. Zhang, H. Yu, D. Wu, H. Ma, H. Li, Q. Wei, Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles. Anal. Biochem. 434(1), 123–127 (2013). doi:10.1016/j.ab.2012.11.012
- M.M. Neves, M.B. González-García, C. Delerue-Matos, A. Costa-García, Multiplexed electrochemical immunosensor for detection of celiac disease serological markers. Sensor Actuat. B 187, 33–39 (2013). doi:10.1016/j.snb.2012.09.019
- D.W. Kimmel, G. LeBlanc, M.E. Meschievitz, D.E. Cliffel, Electrochemical sensors and biosensors. Anal. Chem. 84(2), 685–707 (2011). doi:10.1021/ac202878q
- X. Chen, Y. Wang, J. Zhou, W. Yan, X. Li, J.J. Zhu, Electrochemical impedance immunosensor based on three-dimensionally ordered macroporous gold film. Anal. Chem. 80(6), 2133–2140 (2008). doi:10.1021/ac7021376
- J. Kailashiya, N. Singh, S.K. Singh, V. Agrawal, D. Dash, Graphene oxide-based biosensor for detection of platelet-derived microparticles: a potential tool for thrombus risk identification. Biosens. Bioelectron. 65, 274–280 (2015). doi:10.1016/j.bios.2014.10.056
- M.I. Pividori, S. Alegret, Micro and nanoparticles in biosensing systems for food safety and environmental monitoring. An example of converging technologies. Microchim. Acta 170(3–4), 227–242 (2003). doi:10.1007/s00604-010-0347-8
- L. Babuin, V.C. Vasile, J.A.R. Perez, J.R. Alegria, H.S. Chai, B. Afessa, A.S. Jaffe, Elevated cardiac troponin is an independent risk factor for short-and long-term mortality in medical intensive care unit patients. Crit. Care Med. 36(3), 759–765 (2008). doi:10.1097/CCM.0B013E318164E2E4
- Y. Pan, G.A. Sonn, M.L. Sin, K.E. Mach, M.C. Shih, V. Gau, J.C. Liao, Electrochemical immunosensor detection of urinary lactoferrin in clinical samples for urinary tract infection diagnosis. Biosens. Bioelectron. 26(2), 649–654 (2010). doi:10.1016/j.bios.2010.07.002
- A. Kumar, B.M. Boruah, X.J. Liang, Gold nanoparticles: promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J. Nanomater. 2011, 202187 (2011). doi:10.1155/2011/202187
- J. Wang, Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens. Bioelectron. 21(10), 1887–1892 (2006). doi:10.1016/j.bios.2005.10.027
- D. Tang, R. Yuan, Y. Chai, Y. Fu, J. Dai, Y. Liu, X. Zhong, New amperometric and potentiometric immunosensors based on gold nanoparticles/tris (2, 2′-pyridyl) cobalt (iii) multilayer films for hepatitis B surface antigen determinations. Biosens. Bioelectron. 21(4), 539–548 (2005). doi:10.1016/j.bios.2004.11.024
- A. Zani, S. Laschi, M. Mascini, G. Marrazza, A new electrochemical multiplexed assay for PSA cancer marker detection. Electroanalysis 23(1), 91–99 (2011). doi:10.1002/elan.201000486
- J. Tang, Y. Wang, J. Li, P. Da, J. Geng, G. Zheng, Sensitive enzymatic glucose detection by TiO2 nanowire photoelectrochemical biosensors. J. Mater. Chem. 2(17), 6153–6157 (2014). doi:10.1039/C3TA14173J
- M.S. Veeramani, K.P. Shyam, N.P. Ratchagar, A. Chadha, E. Bhattacharya, Miniaturised silicon biosensors for the detection of triglyceride in blood serum. Anal. Methods 6(6), 1728–1735 (2014). doi:10.1039/c3ay42274g
- X. Cai, X. Gao, L. Wang, Q. Wu, X. Lin, A layer-by-layer assembled and carbon nanotubes/gold nanoparticles-based bienzyme biosensor for cholesterol detection. Sens. Actuat. B 181, 575–583 (2013). doi:10.1016/j.snb.2013.02.050
- X. Luo, J.J. Davis, Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 42(13), 5944–5962 (2013). doi:10.1039/c3cs60077g
- S. Cosnier, Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films: a review. Biosens. Bioelectron. 14(5), 443–456 (1999). doi:10.1016/S0956-5663(99)00024-X
- K.M. Millan, A.J. Spurmanis, S.R. Mikkelsen, Covalent immobilization of DNA onto glassy carbon electrodes. Electroanalysis 4(10), 929–932 (1992). doi:10.1002/elan.1140041003
- E.H. Yoo, S.Y. Lee, Glucose biosensors: an overview of use in clinical practice. Sensors 10(5), 4558–4576 (2010). doi:10.3390/s100504558
- C.S. Shan, H.F. Yang, J.F. Song, D.X. Han, A. Ivaska, L. Niu, Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 81(6), 2378–2382 (2009). doi:10.1021/ac802193c
- X.H. Kang, J. Wang, H. Wu, A.I. Aksay, J. Liu, Y.H. Lin, Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 25(4), 901–905 (2009). doi:10.1016/j.bios.2009.09.004
- L. Zheng, L. Jia, B. Li, B. Situ, Q. Liu, Q. Wang, N. Gan, A sandwich HIV p24 amperometric immunosensor based on a direct gold electroplating-modified electrode. Molecules 17(5), 5988–6000 (2012). doi:10.3390/molecules17055988
- J.A.A. Ho, H.C. Chang, N.Y. Shih, L.C. Wu, Y.F. Chang, C.C. Chen, C. Chou, Diagnostic detection of human lung cancer-associated antigen using a gold nanoparticle-based electrochemical immunosensor. Anal. Chem. 82(14), 5944–5950 (2010). doi:10.1021/ac1001959
- D. Brady, J. Jordaan, Advances in enzyme immobilisation. Biotechnol. Lett. 31(11), 1639–1650 (2009). doi:10.1007/s10529-009-0076-4
- E. Farjami, L. Clima, K. Gothelf, E.E. Ferapontova, “Off–On” electrochemical hairpin-dna-based genosensor for cancer diagnostics. Anal. Chem. 83(5), 1594–1602 (2011). doi:10.1021/ac1032929
- A. Merkoçi, M. Aldavert, S. Marın, S. Alegret, New materials for electrochemical sensing v: nanoparticles for DNA labelling. TrAC-Trend Anal. Chem. 24(4), 341–349 (2005). doi:10.1016/j.trac.2004.11.007
- S. Wang, L. Li, H. Jin, T. Yang, W. Bao, S. Huang, J. Wang, Electrochemical detection of hepatitis B and papilloma virus DNAs using SWCNT array coated with gold nanoparticles. Biosens. Bioelectron. 41, 205–210 (2013). doi:10.1016/j.bios.2012.08.021
- M. Zhou, Y.M. Zhai, S.J. Dong, Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 81(14), 5603–5613 (2009). doi:10.1021/ac900136z
- L. Feng, Y. Chen, J. Ren, X. Qu, A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cell. Biomaterial 32(11), 2930–2937 (2011). doi:10.1016/j.biomaterials.2011.01.002
- P.S. Sharma, A. Pietrzyk-Le, F. D’Souza, W. Kutner, Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 402(10), 3177–3204 (2012). doi:10.1007/s00216-011-5696-6
- Y. Wan, Z. Lin, D. Zhang, Y. Wang, B. Hou, Impedimetric immunosensor doped with reduced graphene sheets fabricated by controllable electrodeposition for the non-labelled detection of bacteria. Biosens. Bioelectron. 26(5), 1959–1964 (2011). doi:10.1016/j.bios.2010.08.008
- K. Kamei, T. Haruyama, M. Mie, Y. Yanagida, M. Aizawa, E. Kobatake, The construction of endothelial cellular biosensing system for the control of blood pressure drugs. Biosens. Bioelectron. 19(9), 1121–1124 (2004). doi:10.1016/j.bios.2003.06.001
- R.S. Skeen, W.S. Kisaalita, B.J. Van Wie, Evaluation of neuron-based sensing with the neurotransmitter serotonin. Biosens. Bioelectron. 5(6), 491–510 (1990). doi:10.1016/0956-5663(90)80037-E
- K.M.L. May, Y. Wang, L.G. Bachas, K.W. Anderson, Development of a whole-cell-based biosensor for detecting histamine as a model toxin. Anal. Chem. 76(14), 4156–4161 (2004). doi:10.1021/ac049810+
- Y.I. Korpan, M.V. Gonchar, N.F. Starodub, A.A. Shul’ga, A.A. Sibirny, A.V. El’skaya, A cell biosensor specific for formaldehyde based on pH-sensitive transistors coupled to methylotrophic yeast cells with genetically adjusted metabolism. Anal. Biochem. 215(2), 216–222 (1993). doi:10.1006/abio.1993.1578
- L. Campanella, G. Favero, D. Mastrofini, M. Tomasetti, Toxicity order of cholanic acids using an immobilized cell biosensor. J. Pharm. Biomed. Anal. 14(8), 1007–1013 (1996). doi:10.1016/0731-7085(95)01709-7
- K.X. Mao, D. Wu, Y. Li, H.M. Ma, Z.Z. Ni, H.Q. Yu, C.N. Luo, Q. Wei, B. Du, Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal. Biochem. 422(1), 22–27 (2012). doi:10.1016/j.ab.2011.12.047
- J.J. Lu, S.Q. Liu, S.G. Ge, M. Yan, J.H. Yu, X.T. Hu, Ultrasensitive electrochemical immunosensor based on Au nanoparticles dotted carbon nanotube–graphene composite and functionalized mesoporous materials. Biosens. Bioelectron. 33(1), 29–35 (2012). doi:10.1016/j.bios.2011.11.054
- A.S. Ahammad, Y.H. Choi, K. Koh, J.H. Kim, J.J. Lee, M. Lee, Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochem. Sci. 6(6), 1906–1916 (2011)
- A. Periyakaruppan, R.P. Gandhiraman, M. Meyyappan, J.E. Koehne, Label-free detection of cardiac troponin-I using carbon nanofiber based nanoelectrode arrays. Anal. Chem. 85(3), 3858–3863 (2013). doi:10.1021/ac302801z
- E. Suprun, T. Bulko, A. Lisitsa, O. Gnedenko, A. Ivanov, V. Shumyantseva, A. Archakov, Electrochemical nanobiosensor for express diagnosis of acute myocardial infarction in undiluted plasma. Biosens. Bioelectron. 25(7), 1694–1698 (2010). doi:10.1016/j.bios.2009.12.009
- G.S. Wilson, R. Gifford, Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 20(12), 2388–2403 (2005). doi:10.1016/j.bios.2004.12.003
- H. Wu, J. Wang, X. Kang, C. Wang, D. Wang, J. Liu, Y. Lin, Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 80(1), 403–406 (2009). doi:10.1016/j.talanta.2009.06.054
- J. Bolinder, U. Ungerstedt, P. Arner, Long-term continuous glucose monitoring with microdialysis in ambulatory insulin-dependent diabetic patients. Lancet 342(8879), 1080–1085 (1993). doi:10.1016/0140-6736(93)92063-Y
- U. Fischer, R. Ertle, P. Abel, K. Rebrin, E. Brunstein, H.H. Von Dorsche, E.J. Freyse, Assessment of subcutaneous glucose concentration: validation of the wick technique as a reference for implanted electrochemical sensors in normal and diabetic dogs. Diabetologia 32(12), 940–945 (1987). doi:10.1007/BF00295878
- B. Aussedat, M. Dupire-Angel, R. Gifford, J.C. Klein, G.S. Wilson, G. Reach, Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am. J. Physiol. Endocrinol. Metab. 278(4), E716–E728 (2000)
- D.J. Claremont, E. Sambrook, C. Penton, J.C. Pickup, Subcutaneous implantation of a ferrocene-mediated glucose sensor in pigs. Diabetologia 29(11), 817–821 (1986). doi:10.1007/BF00873223
- J. Pickup, Developing glucose sensors for in vivo use. Trends Biotechnol. 11(7), 285–291 (1993). doi:10.1016/0167-7799(93)90016-3
- J.J. Mastrototaro, The MiniMed continuous glucose monitoring system. Diabetes Technol. Ther. 2(1), 13–18 (2000). doi:10.1089/15209150050214078
- T.M. Gross, B.W. Bode, D. Einhorn, D.M. Kayne, J.H. Reed, N.H. White, J.J. Mastrototaro, Performance evaluation of the MiniMed® continuous glucose monitoring system during patient home use. Diabetes Technol. Ther. 2(1), 49–56 (2000). doi:10.1089/152091500316737
- M.J. Tierney, J.A. Tamada, R.O. Potts, L. Jovanovic, S. Garg, Cygnus Research Team, clinical evaluation of the glucowatch® biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 16(9), 621–629 (2001). doi:10.1016/S0956-5663(01)00189-0
- Y. Hu, K.M. Mitchell, F.N. Albahadily, E.K. Michaelis, G.S. Wilson, Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 659(1), 117–125 (1994). doi:10.1016/0006-8993(94)90870-2
- C.J. Griessenauer, S.Y. Chang, S.J. Tye, C.J. Kimble, K.E. Bennet, P.A. Garris, K.H. Lee, Wireless instantaneous neurotransmitter concentration system: electrochemical monitoring of serotonin using fast-scan cyclic voltammetry—a proof-of-principle study. J. Neurosurg. 113(3), 656–665 (2010). doi:10.3171/2010.3.JNS091627
- F. Bedioui, N. Villeneuve, Electrochemical nitric oxide sensors for biological samples–principle, selected examples and applications. Electroanalysis 15(1), 5–18 (2003). doi:10.1002/elan.200390006
- A. Nemiroski, D.C. Christodouleas, J.W. Hennek, A.A. Kumar, E.J. Maxwell, M.T. Fernández-Abedul, G.M. Whitesides, Universal mobile electrochemical detector designed for use in resource-limited applications. PNAS 111(33), 11984–11989 (2014). doi:10.1073/pnas.1405679111
- W. Yantasee, K. Hongsirikarn, C.L. Warner, D. Choi, T. Sangvanich, M.B. Toloczko, C. Timchalk, Direct detection of Pb in urine and Cd, Pb, Cu, and Ag in natural waters using electrochemical sensors immobilized with DMSA functionalized magnetic nanoparticles. Analyst 133(3), 348–355 (2008). doi:10.1039/b711199a
- J. Kudr, H.V. Nguyen, J. Gumulec, L. Nejdl, I. Blazkova, B. Ruttkay-Nedecky, R. Kizek, Simultaneous automatic electrochemical detection of zinc, cadmium, copper and lead ions in environmental samples using a thin-film mercury electrode and an artificial neural network. Sensors 15, 592–610 (2015). doi:10.3390/s150100592
- R. Kensova, D. Hynek, J. Kynicky, M. Konecna, T. Eckschlager, V. Adam, R. Kizek, Determination of metal ions in the plasma of children with tumour diseases by differential pulse voltammetry. Int. J. Electrochem. Sci. 9, 4675–4691 (2014)
- T.Z. Liu, D. Lai, J.D. Osterloh, Indium as internal standard in square wave anodic stripping analysis of lead in blood with microelectrode arrays. Anal. Chem. 69(17), 3539–3543 (1997). doi:10.1021/ac9612483
- B.V. Chikkaveeraiah, A.A. Bhirde, N.Y. Morgan, H.S. Eden, X. Chen, Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 6(8), 6546–6561 (2012). doi:10.1021/nn3023969
- P.A. Muller, K.H. Vousden, P53 mutations in cancer. Nat. Cell Boil. 15(1), 2–8 (2013). doi:10.1038/ncb2641
- Y. Hu, P. Zuo, B.C. Ye, Label-free electrochemical impedance spectroscopy biosensor for direct detection of cancer cells based on the interaction between carbohydrate and lectin. Biosens. Bioelectron. 43, 79–83 (2013). doi:10.1016/j.bios.2012.11.028
- C.B. Jacobs, M.J. Peairs, B.J. Venton, Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 662(2), 105–127 (2010). doi:10.1016/j.aca.2010.01.009
- K.A. Mahmoud, S. Hrapovic, J.H. Luong, Picomolar detection of protease using peptide/single walled carbon nanotube/gold nanoparticle-modified electrode. ACS Nano 2(5), 1051–1057 (2008). doi:10.1021/nn8000774
- K.S. Huang, W.T. Chen, S.J. Lee, C.H. Yeh, T.C. Chang, H.P. Lin, Y.C. Lin, A novel and efficient immunoassay: using electro-microchip, gold nanoparticle and silver enhancement. In Industrial Electronics Society 2007. IECON 2007. 33rd annual conference of the IEEE 2966–2969 (2007). doi:10.1109/iecon.2007.4459931
- W. Yantasee, Y. Lin, K. Hongsirikarn, G.E. Fryxell, R. Addleman, C. Timchalk, Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors. Environ. Health Persp. 115(12), 1683–1690 (2008). doi:10.1289/ehp.10190
- P. Jothimuthu, R.A. Wilson, J. Herren, E.N. Haynes, W.R. Heineman, I. Papautsky, Lab-on-a-chip sensor for detection of highly electronegative heavy metals by anodic stripping voltammetry. Biomed. Microdevices 13(4), 695–703 (2011). doi:10.1007/s10544-011-9539-1
- C.C. Yang, A.S. Kumar, J.M. Zen, Precise blood lead analysis using a combined internal standard and standard addition approach with disposable screen-printed electrodes. Anal. Biochem. 338(2), 278–283 (2005). doi:10.1016/j.ab.2004.12.015
- J. Kruusma, L. Nei, J.L. Hardcastle, R.G. Compton, E. Lust, H. Keis, Sonoelectroanalysis: anodic stripping voltammetric determination of cadmium in whole human blood. Electroanalysis 16(5), 399–403 (2004). doi:10.1002/elan.200302834
- W. Yantasee, C. Timchalk, Y. Lin, Microanalyzer for biomonitoring lead (Pb) in blood and urine. Anal. Bioanal. Chem. 387(1), 335–341 (2007). doi:10.1007/s00216-006-0940-1
References
A. Hulanicki, S. Glab, F.O.L.K.E. Ingman, Chemical sensors: definitions and classification. Pure Appl. Chem. 63(9), 1247–1250 (1991). doi:10.1351/pac199163091247
S.K. Arya, S. Saha, J.E. Ramirez-Vick, V. Gupta, S. Bhansali, S.P. Singh, Recent advances in ZnO nanostructures and thin films for biosensor applications: review. Anal. Chim. Acta 737, 1–21 (2012). doi:10.1016/j.aca.2012.05.048
J. Wang, Sol–gel materials for electrochemical biosensors. Anal. Chim. Acta 399(1), 21–27 (1999). doi:10.1016/S0003-2670(99)00572-3
J. Wang, D. Xu, A.N. Kawde, R. Polsky, Metal nanoparticle-based electrochemical stripping potentiometric detection of DNA hybridization. Anal. Chem. 73(22), 5576–5581 (2001). doi:10.1021/ac0107148
S. Zhang, N. Wang, H. Yu, Y. Niu, C. Sun, Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 67(1), 15–22 (2005). doi:10.1016/j.bioelechem.2004.12.002
J. Wang, Carbon–nanotube based electrochemical biosensors: a review. Electroanalysis 17(1), 7–14 (2005). doi:10.1002/elan.200403113
J. Wang, Nanomaterial-based electrochemical biosensors. Analyst 130(4), 421–426 (2005). doi:10.1039/b414248a
A. Zhu, Q. Qu, X. Shao, B. Kong, Y. Tian, Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew. Chem. 124(29), 7297–7301 (2012). doi:10.1002/ange.201109089
K.J. Cash, H.A. Clark, Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 16(12), 584–593 (2010). doi:10.1016/j.molmed.2010.08.002
S. Solé, A. Merkoci, S. Alegret, New materials for electrochemical sensing III beads. TrAC-Trend Anal. Chem. 20(2), 102–110 (2001). doi:10.1016/s0165-9936(00)00059-5
H. Wang, Y. Zhang, H. Yu, D. Wu, H. Ma, H. Li, Q. Wei, Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles. Anal. Biochem. 434(1), 123–127 (2013). doi:10.1016/j.ab.2012.11.012
M.M. Neves, M.B. González-García, C. Delerue-Matos, A. Costa-García, Multiplexed electrochemical immunosensor for detection of celiac disease serological markers. Sensor Actuat. B 187, 33–39 (2013). doi:10.1016/j.snb.2012.09.019
D.W. Kimmel, G. LeBlanc, M.E. Meschievitz, D.E. Cliffel, Electrochemical sensors and biosensors. Anal. Chem. 84(2), 685–707 (2011). doi:10.1021/ac202878q
X. Chen, Y. Wang, J. Zhou, W. Yan, X. Li, J.J. Zhu, Electrochemical impedance immunosensor based on three-dimensionally ordered macroporous gold film. Anal. Chem. 80(6), 2133–2140 (2008). doi:10.1021/ac7021376
J. Kailashiya, N. Singh, S.K. Singh, V. Agrawal, D. Dash, Graphene oxide-based biosensor for detection of platelet-derived microparticles: a potential tool for thrombus risk identification. Biosens. Bioelectron. 65, 274–280 (2015). doi:10.1016/j.bios.2014.10.056
M.I. Pividori, S. Alegret, Micro and nanoparticles in biosensing systems for food safety and environmental monitoring. An example of converging technologies. Microchim. Acta 170(3–4), 227–242 (2003). doi:10.1007/s00604-010-0347-8
L. Babuin, V.C. Vasile, J.A.R. Perez, J.R. Alegria, H.S. Chai, B. Afessa, A.S. Jaffe, Elevated cardiac troponin is an independent risk factor for short-and long-term mortality in medical intensive care unit patients. Crit. Care Med. 36(3), 759–765 (2008). doi:10.1097/CCM.0B013E318164E2E4
Y. Pan, G.A. Sonn, M.L. Sin, K.E. Mach, M.C. Shih, V. Gau, J.C. Liao, Electrochemical immunosensor detection of urinary lactoferrin in clinical samples for urinary tract infection diagnosis. Biosens. Bioelectron. 26(2), 649–654 (2010). doi:10.1016/j.bios.2010.07.002
A. Kumar, B.M. Boruah, X.J. Liang, Gold nanoparticles: promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J. Nanomater. 2011, 202187 (2011). doi:10.1155/2011/202187
J. Wang, Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens. Bioelectron. 21(10), 1887–1892 (2006). doi:10.1016/j.bios.2005.10.027
D. Tang, R. Yuan, Y. Chai, Y. Fu, J. Dai, Y. Liu, X. Zhong, New amperometric and potentiometric immunosensors based on gold nanoparticles/tris (2, 2′-pyridyl) cobalt (iii) multilayer films for hepatitis B surface antigen determinations. Biosens. Bioelectron. 21(4), 539–548 (2005). doi:10.1016/j.bios.2004.11.024
A. Zani, S. Laschi, M. Mascini, G. Marrazza, A new electrochemical multiplexed assay for PSA cancer marker detection. Electroanalysis 23(1), 91–99 (2011). doi:10.1002/elan.201000486
J. Tang, Y. Wang, J. Li, P. Da, J. Geng, G. Zheng, Sensitive enzymatic glucose detection by TiO2 nanowire photoelectrochemical biosensors. J. Mater. Chem. 2(17), 6153–6157 (2014). doi:10.1039/C3TA14173J
M.S. Veeramani, K.P. Shyam, N.P. Ratchagar, A. Chadha, E. Bhattacharya, Miniaturised silicon biosensors for the detection of triglyceride in blood serum. Anal. Methods 6(6), 1728–1735 (2014). doi:10.1039/c3ay42274g
X. Cai, X. Gao, L. Wang, Q. Wu, X. Lin, A layer-by-layer assembled and carbon nanotubes/gold nanoparticles-based bienzyme biosensor for cholesterol detection. Sens. Actuat. B 181, 575–583 (2013). doi:10.1016/j.snb.2013.02.050
X. Luo, J.J. Davis, Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 42(13), 5944–5962 (2013). doi:10.1039/c3cs60077g
S. Cosnier, Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films: a review. Biosens. Bioelectron. 14(5), 443–456 (1999). doi:10.1016/S0956-5663(99)00024-X
K.M. Millan, A.J. Spurmanis, S.R. Mikkelsen, Covalent immobilization of DNA onto glassy carbon electrodes. Electroanalysis 4(10), 929–932 (1992). doi:10.1002/elan.1140041003
E.H. Yoo, S.Y. Lee, Glucose biosensors: an overview of use in clinical practice. Sensors 10(5), 4558–4576 (2010). doi:10.3390/s100504558
C.S. Shan, H.F. Yang, J.F. Song, D.X. Han, A. Ivaska, L. Niu, Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 81(6), 2378–2382 (2009). doi:10.1021/ac802193c
X.H. Kang, J. Wang, H. Wu, A.I. Aksay, J. Liu, Y.H. Lin, Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 25(4), 901–905 (2009). doi:10.1016/j.bios.2009.09.004
L. Zheng, L. Jia, B. Li, B. Situ, Q. Liu, Q. Wang, N. Gan, A sandwich HIV p24 amperometric immunosensor based on a direct gold electroplating-modified electrode. Molecules 17(5), 5988–6000 (2012). doi:10.3390/molecules17055988
J.A.A. Ho, H.C. Chang, N.Y. Shih, L.C. Wu, Y.F. Chang, C.C. Chen, C. Chou, Diagnostic detection of human lung cancer-associated antigen using a gold nanoparticle-based electrochemical immunosensor. Anal. Chem. 82(14), 5944–5950 (2010). doi:10.1021/ac1001959
D. Brady, J. Jordaan, Advances in enzyme immobilisation. Biotechnol. Lett. 31(11), 1639–1650 (2009). doi:10.1007/s10529-009-0076-4
E. Farjami, L. Clima, K. Gothelf, E.E. Ferapontova, “Off–On” electrochemical hairpin-dna-based genosensor for cancer diagnostics. Anal. Chem. 83(5), 1594–1602 (2011). doi:10.1021/ac1032929
A. Merkoçi, M. Aldavert, S. Marın, S. Alegret, New materials for electrochemical sensing v: nanoparticles for DNA labelling. TrAC-Trend Anal. Chem. 24(4), 341–349 (2005). doi:10.1016/j.trac.2004.11.007
S. Wang, L. Li, H. Jin, T. Yang, W. Bao, S. Huang, J. Wang, Electrochemical detection of hepatitis B and papilloma virus DNAs using SWCNT array coated with gold nanoparticles. Biosens. Bioelectron. 41, 205–210 (2013). doi:10.1016/j.bios.2012.08.021
M. Zhou, Y.M. Zhai, S.J. Dong, Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 81(14), 5603–5613 (2009). doi:10.1021/ac900136z
L. Feng, Y. Chen, J. Ren, X. Qu, A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cell. Biomaterial 32(11), 2930–2937 (2011). doi:10.1016/j.biomaterials.2011.01.002
P.S. Sharma, A. Pietrzyk-Le, F. D’Souza, W. Kutner, Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 402(10), 3177–3204 (2012). doi:10.1007/s00216-011-5696-6
Y. Wan, Z. Lin, D. Zhang, Y. Wang, B. Hou, Impedimetric immunosensor doped with reduced graphene sheets fabricated by controllable electrodeposition for the non-labelled detection of bacteria. Biosens. Bioelectron. 26(5), 1959–1964 (2011). doi:10.1016/j.bios.2010.08.008
K. Kamei, T. Haruyama, M. Mie, Y. Yanagida, M. Aizawa, E. Kobatake, The construction of endothelial cellular biosensing system for the control of blood pressure drugs. Biosens. Bioelectron. 19(9), 1121–1124 (2004). doi:10.1016/j.bios.2003.06.001
R.S. Skeen, W.S. Kisaalita, B.J. Van Wie, Evaluation of neuron-based sensing with the neurotransmitter serotonin. Biosens. Bioelectron. 5(6), 491–510 (1990). doi:10.1016/0956-5663(90)80037-E
K.M.L. May, Y. Wang, L.G. Bachas, K.W. Anderson, Development of a whole-cell-based biosensor for detecting histamine as a model toxin. Anal. Chem. 76(14), 4156–4161 (2004). doi:10.1021/ac049810+
Y.I. Korpan, M.V. Gonchar, N.F. Starodub, A.A. Shul’ga, A.A. Sibirny, A.V. El’skaya, A cell biosensor specific for formaldehyde based on pH-sensitive transistors coupled to methylotrophic yeast cells with genetically adjusted metabolism. Anal. Biochem. 215(2), 216–222 (1993). doi:10.1006/abio.1993.1578
L. Campanella, G. Favero, D. Mastrofini, M. Tomasetti, Toxicity order of cholanic acids using an immobilized cell biosensor. J. Pharm. Biomed. Anal. 14(8), 1007–1013 (1996). doi:10.1016/0731-7085(95)01709-7
K.X. Mao, D. Wu, Y. Li, H.M. Ma, Z.Z. Ni, H.Q. Yu, C.N. Luo, Q. Wei, B. Du, Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal. Biochem. 422(1), 22–27 (2012). doi:10.1016/j.ab.2011.12.047
J.J. Lu, S.Q. Liu, S.G. Ge, M. Yan, J.H. Yu, X.T. Hu, Ultrasensitive electrochemical immunosensor based on Au nanoparticles dotted carbon nanotube–graphene composite and functionalized mesoporous materials. Biosens. Bioelectron. 33(1), 29–35 (2012). doi:10.1016/j.bios.2011.11.054
A.S. Ahammad, Y.H. Choi, K. Koh, J.H. Kim, J.J. Lee, M. Lee, Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochem. Sci. 6(6), 1906–1916 (2011)
A. Periyakaruppan, R.P. Gandhiraman, M. Meyyappan, J.E. Koehne, Label-free detection of cardiac troponin-I using carbon nanofiber based nanoelectrode arrays. Anal. Chem. 85(3), 3858–3863 (2013). doi:10.1021/ac302801z
E. Suprun, T. Bulko, A. Lisitsa, O. Gnedenko, A. Ivanov, V. Shumyantseva, A. Archakov, Electrochemical nanobiosensor for express diagnosis of acute myocardial infarction in undiluted plasma. Biosens. Bioelectron. 25(7), 1694–1698 (2010). doi:10.1016/j.bios.2009.12.009
G.S. Wilson, R. Gifford, Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 20(12), 2388–2403 (2005). doi:10.1016/j.bios.2004.12.003
H. Wu, J. Wang, X. Kang, C. Wang, D. Wang, J. Liu, Y. Lin, Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 80(1), 403–406 (2009). doi:10.1016/j.talanta.2009.06.054
J. Bolinder, U. Ungerstedt, P. Arner, Long-term continuous glucose monitoring with microdialysis in ambulatory insulin-dependent diabetic patients. Lancet 342(8879), 1080–1085 (1993). doi:10.1016/0140-6736(93)92063-Y
U. Fischer, R. Ertle, P. Abel, K. Rebrin, E. Brunstein, H.H. Von Dorsche, E.J. Freyse, Assessment of subcutaneous glucose concentration: validation of the wick technique as a reference for implanted electrochemical sensors in normal and diabetic dogs. Diabetologia 32(12), 940–945 (1987). doi:10.1007/BF00295878
B. Aussedat, M. Dupire-Angel, R. Gifford, J.C. Klein, G.S. Wilson, G. Reach, Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am. J. Physiol. Endocrinol. Metab. 278(4), E716–E728 (2000)
D.J. Claremont, E. Sambrook, C. Penton, J.C. Pickup, Subcutaneous implantation of a ferrocene-mediated glucose sensor in pigs. Diabetologia 29(11), 817–821 (1986). doi:10.1007/BF00873223
J. Pickup, Developing glucose sensors for in vivo use. Trends Biotechnol. 11(7), 285–291 (1993). doi:10.1016/0167-7799(93)90016-3
J.J. Mastrototaro, The MiniMed continuous glucose monitoring system. Diabetes Technol. Ther. 2(1), 13–18 (2000). doi:10.1089/15209150050214078
T.M. Gross, B.W. Bode, D. Einhorn, D.M. Kayne, J.H. Reed, N.H. White, J.J. Mastrototaro, Performance evaluation of the MiniMed® continuous glucose monitoring system during patient home use. Diabetes Technol. Ther. 2(1), 49–56 (2000). doi:10.1089/152091500316737
M.J. Tierney, J.A. Tamada, R.O. Potts, L. Jovanovic, S. Garg, Cygnus Research Team, clinical evaluation of the glucowatch® biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 16(9), 621–629 (2001). doi:10.1016/S0956-5663(01)00189-0
Y. Hu, K.M. Mitchell, F.N. Albahadily, E.K. Michaelis, G.S. Wilson, Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 659(1), 117–125 (1994). doi:10.1016/0006-8993(94)90870-2
C.J. Griessenauer, S.Y. Chang, S.J. Tye, C.J. Kimble, K.E. Bennet, P.A. Garris, K.H. Lee, Wireless instantaneous neurotransmitter concentration system: electrochemical monitoring of serotonin using fast-scan cyclic voltammetry—a proof-of-principle study. J. Neurosurg. 113(3), 656–665 (2010). doi:10.3171/2010.3.JNS091627
F. Bedioui, N. Villeneuve, Electrochemical nitric oxide sensors for biological samples–principle, selected examples and applications. Electroanalysis 15(1), 5–18 (2003). doi:10.1002/elan.200390006
A. Nemiroski, D.C. Christodouleas, J.W. Hennek, A.A. Kumar, E.J. Maxwell, M.T. Fernández-Abedul, G.M. Whitesides, Universal mobile electrochemical detector designed for use in resource-limited applications. PNAS 111(33), 11984–11989 (2014). doi:10.1073/pnas.1405679111
W. Yantasee, K. Hongsirikarn, C.L. Warner, D. Choi, T. Sangvanich, M.B. Toloczko, C. Timchalk, Direct detection of Pb in urine and Cd, Pb, Cu, and Ag in natural waters using electrochemical sensors immobilized with DMSA functionalized magnetic nanoparticles. Analyst 133(3), 348–355 (2008). doi:10.1039/b711199a
J. Kudr, H.V. Nguyen, J. Gumulec, L. Nejdl, I. Blazkova, B. Ruttkay-Nedecky, R. Kizek, Simultaneous automatic electrochemical detection of zinc, cadmium, copper and lead ions in environmental samples using a thin-film mercury electrode and an artificial neural network. Sensors 15, 592–610 (2015). doi:10.3390/s150100592
R. Kensova, D. Hynek, J. Kynicky, M. Konecna, T. Eckschlager, V. Adam, R. Kizek, Determination of metal ions in the plasma of children with tumour diseases by differential pulse voltammetry. Int. J. Electrochem. Sci. 9, 4675–4691 (2014)
T.Z. Liu, D. Lai, J.D. Osterloh, Indium as internal standard in square wave anodic stripping analysis of lead in blood with microelectrode arrays. Anal. Chem. 69(17), 3539–3543 (1997). doi:10.1021/ac9612483
B.V. Chikkaveeraiah, A.A. Bhirde, N.Y. Morgan, H.S. Eden, X. Chen, Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 6(8), 6546–6561 (2012). doi:10.1021/nn3023969
P.A. Muller, K.H. Vousden, P53 mutations in cancer. Nat. Cell Boil. 15(1), 2–8 (2013). doi:10.1038/ncb2641
Y. Hu, P. Zuo, B.C. Ye, Label-free electrochemical impedance spectroscopy biosensor for direct detection of cancer cells based on the interaction between carbohydrate and lectin. Biosens. Bioelectron. 43, 79–83 (2013). doi:10.1016/j.bios.2012.11.028
C.B. Jacobs, M.J. Peairs, B.J. Venton, Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 662(2), 105–127 (2010). doi:10.1016/j.aca.2010.01.009
K.A. Mahmoud, S. Hrapovic, J.H. Luong, Picomolar detection of protease using peptide/single walled carbon nanotube/gold nanoparticle-modified electrode. ACS Nano 2(5), 1051–1057 (2008). doi:10.1021/nn8000774
K.S. Huang, W.T. Chen, S.J. Lee, C.H. Yeh, T.C. Chang, H.P. Lin, Y.C. Lin, A novel and efficient immunoassay: using electro-microchip, gold nanoparticle and silver enhancement. In Industrial Electronics Society 2007. IECON 2007. 33rd annual conference of the IEEE 2966–2969 (2007). doi:10.1109/iecon.2007.4459931
W. Yantasee, Y. Lin, K. Hongsirikarn, G.E. Fryxell, R. Addleman, C. Timchalk, Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors. Environ. Health Persp. 115(12), 1683–1690 (2008). doi:10.1289/ehp.10190
P. Jothimuthu, R.A. Wilson, J. Herren, E.N. Haynes, W.R. Heineman, I. Papautsky, Lab-on-a-chip sensor for detection of highly electronegative heavy metals by anodic stripping voltammetry. Biomed. Microdevices 13(4), 695–703 (2011). doi:10.1007/s10544-011-9539-1
C.C. Yang, A.S. Kumar, J.M. Zen, Precise blood lead analysis using a combined internal standard and standard addition approach with disposable screen-printed electrodes. Anal. Biochem. 338(2), 278–283 (2005). doi:10.1016/j.ab.2004.12.015
J. Kruusma, L. Nei, J.L. Hardcastle, R.G. Compton, E. Lust, H. Keis, Sonoelectroanalysis: anodic stripping voltammetric determination of cadmium in whole human blood. Electroanalysis 16(5), 399–403 (2004). doi:10.1002/elan.200302834
W. Yantasee, C. Timchalk, Y. Lin, Microanalyzer for biomonitoring lead (Pb) in blood and urine. Anal. Bioanal. Chem. 387(1), 335–341 (2007). doi:10.1007/s00216-006-0940-1