Fabrication and Applications of Micro/Nanostructured Devices for Tissue Engineering
Corresponding Author: Tania Limongi
Nano-Micro Letters,
Vol. 9 No. 1 (2017), Article Number: 1
Abstract
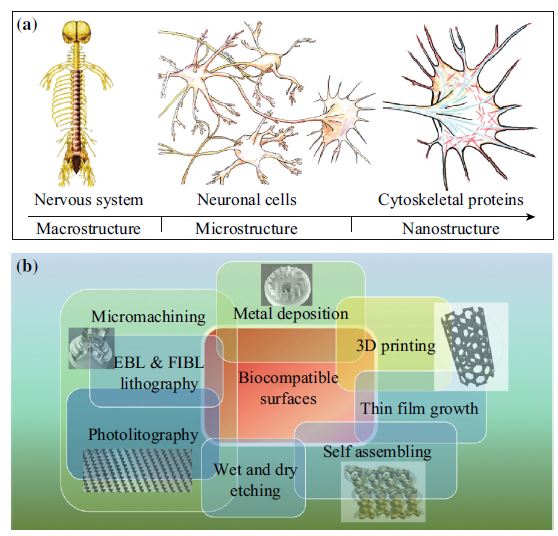
Nanotechnology allows the realization of new materials and devices with basic structural unit in the range of 1–100 nm and characterized by gaining control at the atomic, molecular, and supramolecular level. Reducing the dimensions of a material into the nanoscale range usually results in the change of its physiochemical properties such as reactivity, crystallinity, and solubility. This review treats the convergence of last research news at the interface of nanostructured biomaterials and tissue engineering for emerging biomedical technologies such as scaffolding and tissue regeneration. The present review is organized into three main sections. The introduction concerns an overview of the increasing utility of nanostructured materials in the field of tissue engineering. It elucidates how nanotechnology, by working in the submicron length scale, assures the realization of a biocompatible interface that is able to reproduce the physiological cell–matrix interaction. The second, more technical section, concerns the design and fabrication of biocompatible surface characterized by micro- and submicroscale features, using microfabrication, nanolithography, and miscellaneous nanolithographic techniques. In the last part, we review the ongoing tissue engineering application of nanostructured materials and scaffolds in different fields such as neurology, cardiology, orthopedics, and skin tissue regeneration.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- J. Hwang, Y. Jeong, J.M. Park, K.H. Lee, J.W. Hong, J. Choi, Biomimetics: forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 8(10), 5701–5713 (2015). doi:10.2147/IJN.S83642
- M.J. Webber, E.A. Appel, E.W. Meijer, R. Langer, Supramolecular biomaterials. Nat. Mater. 15(1), 13–26 (2015). doi:10.1038/nmat4474
- T.U. Luu, S.C. Gott, B.W. Woo, M.P. Rao, W.F. Liu, Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl. Mater. Interfaces 7(51), 28665–28672 (2015). doi:10.1021/acsami.5b10589
- N. Mauro, A. Manfredi, E. Ranucci, P. Procacci, M. Laus, D. Antonioli, C. Mantovani, V. Magnaghi, P. Ferruti, Degradable poly(amidoamine) hydrogels as scaffolds for in vitro culturing of peripheral nervous system cells. Macromol. Biosci. 13(3), 332–347 (2013). doi:10.1002/mabi.201200354
- W. Zhu, C. O’Brien, J.R. O’Brien, L.G. Zhang, 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine 9(6), 859–875 (2014). doi:10.2217/nnm.14.36
- P. Haisheng, L. Xunpei, W. Ran, J. Feng, D. Liang, W. Qun, Emerging nanostructured materials for musculoskeletal tissue engineering. J. Mater. Chem. B 2(38), 6435–6461 (2014). doi:10.1039/c4tb00344f
- H. Park, C. Cannizzaro, G. Vunjak-Novakovic, R. Langer, C.A. Vacanti, O.C. Farokhzad, Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 13(8), 1867–1877 (2007). doi:10.1089/ten.2006.0198
- B.N. Brown, B.D. Ratner, S.B. Goodman, S. Amar, S.F. Badylak, Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33(15), 3792–3802 (2012). doi:10.1016/j.biomaterials.2012.02.034
- B.D. Ratner, S.J. Bryant, Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 6, 41–75 (2004). doi:10.1146/annurev.bioeng.6.040803.140027
- M. Veiseh, A. Nikjoo, E.A. Turley, M.J. Bissell, Nanotechnology and regenerative engineering: the scaffold: from microenvironment to nanoenvironment: ultrastructure and function of extracellular matrix, ed. by C.T. Laurencin, L.S. Nair (CRC Press, Boca Raton, 2015), pp 39–62
- F. Gattazzo, A. Urciuolo, P. Bonaldo, Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 1840(8), 2506–2519 (2014). doi:10.1016/j.bbagen.2014.01.010
- R.G. Flemming, C.J. Murphy, G.A. Abrams, S.L. Goodman, P.F. Nealey, Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 20(6), 573–588 (1999)
- F. Gentile, R. La Rocca, G. Marinaro, A. Nicastri, A. Toma et al., Differential cell adhesion on mesoporous silicon substrates. ACS Appl. Mater. Interfaces 4(6), 2903–2911 (2012). doi:10.1021/am300519a
- F. Gentile, L. Tirinato, E. Battista, F. Causa, C. Liberale, E.M. di Fabrizio, P. Decuzzi, Cells preferentially grow on rough substrates. Biomaterials 31(28), 7205–7212 (2010). doi:10.1016/j.biomaterials.2010.06.016
- T. Limongi, F. Cesca, F. Gentile, R. Marotta, R. Ruffilli et al., Nanostructured superhydrophobic substrates trigger the development of 3d neuronal networks. Small 9(3), 402–412 (2013). doi:10.1002/smll.201201377
- F. Cesca, T. Limongi, A. Accardo, A. Rocchi, M. Orlando, V. Shalabaeva, E. Di Fabrizio, F. Benfenati, Fabrication of biocompatible free-standing nanopatterned films for primary neuronal cultures. RSC Adv. 4(86), 45696–45702 (2014). doi:10.1039/c4ra08361j
- T. Limongi, R. Schipani, A. Di Vito, A. Giugni, M. Francardi et al., Photolithography and micromolding techniques for the realization of 3D polycaprolactone scaffolds for tissue engineering applications. Microelectron. Eng. 141, 135–139 (2015). doi:10.1016/j.mee.2015.02.030
- T. Limongi, E. Miele, V. Shalabaeva, R. La Rocca, R. Schipani et al., Development, characterization and cell cultural response of 3d biocompatible micro-patterned poly-ε-caprolactone scaffolds designed and fabricated integrating lithography and micromolding fabrication techniques. J. Tissue Sci. Eng. 6(1), 5 (2015). doi:10.4172/2157-7552.1000145
- K.M. Ainslie, T.A. Desai, Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip 8(11), 1864–1878 (2008). doi:10.1039/b806446f
- C.W. Park, Y.S. Rhee, S.H. Park, S.D. Danh, S.H. Ahn, S.C. Chi, E.S. Park, In vitro/in vivo evaluation of NCDS-micro-fabricated biodegradable implant. Arch. Pharm. Res. 33(3), 427–432 (2010). doi:10.1007/s12272-010-0312-4
- A.S. Curtis, C.D. Wilkinson, J. Crossan, C. Broadley, H. Darmani, K.K. Johal, H. Jorgensen, W. Monaghan, An in vivo microfabricated scaffold for tendon repair. Eur. Cell Mater. 9, 50–57 (2005)
- D. Shi, X. Xu, Y. Ye, K. Song, Y. Cheng, J. Di, Q. Hu, J. Li, H. Ju, Q. Jiang, Z. Gu, Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 10(1), 1292–1299 (2016). doi:10.1021/acsnano.5b06663
- K. Baranes, M. Shevach, O. Shefi, T. Dvir, Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 16(5), 2916–2920 (2015). doi:10.1021/acs.nanolett.5b04033
- E.L. Hopley, S. Salmasi, D.M. Kalaskar, A.M. Seifalian, Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 32(5), 1000–1014 (2014). doi:10.1016/j.biotechadv.2014.05.003
- A. Childs, U.D. Hemraz, N.J. Castro, H. Fenniri, L.G. Zhang, Novel biologically-inspired rosette nanotube PLLA scaffolds for improving human mesenchymal stem cell chondrogenic differentiation. Biomed. Mater. 8(6), 065003 (2013). doi:10.1088/1748-6041/8/6/065003
- Y.C. Shin, J.H. Lee, M.J. Kim, S.W. Hong, B. Kim, J.K. Hyun, Y.S. Choi, J.C. Park, D.W. Han, Stimulating effect of graphene oxide on myogenesis of C2C12 myoblasts on RGD peptide-decorated PLGA nanofiber matrices. J. Biol. Eng. 9, 22 (2015). doi:10.1186/s13036-015-0020-1
- A. Raspa, A. Marchini, R. Pugliese, M. Mauri, M. Maleki, R. Vasita, F. Gelain, A biocompatibility study of new nanofibrous scaffolds for nervous system regeneration. Nanoscale 8(1), 253–265 (2015). doi:10.1039/C5NR03698D
- J.M. Szymanski, Q. Jallerat, A.W. Feinberg, ECM protein nanofibers and nanostructures engineered using surface-initiated assembly. J. Vis. Exp. 86, e51176 (2014). doi:10.3791/51176
- C. Mota, S. Danti, D. D’Alessandro, L. Trombi, C. Ricci et al., Multiscale fabrication of biomimetic scaffolds for tympanic membrane tissue engineering. Biofabrication 7(2), 025005 (2015). doi:10.1088/1758-5090/7/2/025005
- E. Zanchetta, E. Guidi, G. Della Giustina, M. Sorgato, M. Krampera et al., Injection molded polymeric micropatterns for bone regeneration study. ACS Appl. Mater. Interfaces 7(13), 7273–7281 (2015). doi:10.1021/acsami.5b00481
- R.E. McMahon, X. Qu, A.C. Jimenez-Vergara, C.A. Bashur, S.A. Guelcher, A.S. Goldstein, M.S. Hahn, Hydrogel-electrospun mesh composites for coronary artery bypass grafts. Tissue Eng. C 17(4), 451–461 (2011). doi:10.1089/ten.tec.2010.0427
- M.J. Lima, V.M. Correlo, R.L. Reis, Micro/nano replication and 3D assembling techniques for scaffold fabrication. Mater. Sci. Eng. Part C 42, 615–621 (2014). doi:10.1016/j.msec.2014.05.064
- V. Raffa, O. Vittorio, V. Pensabene, A. Menciassi, P. Dario, FIB-nanostructured surfaces and investigation of Bio/nonbio interactions at the nanoscale. IEEE Trans. Nanobiosci. 7(1), 1–10 (2008). doi:10.1109/TNB.2008.2000143
- F. De Angelis, C. Liberale, M.L. Coluccio, G. Cojoc, E. Di Fabrizio, Emerging fabrication techniques for 3D nano-structuring in plasmonics and single molecule studies. Nanoscale 3(7), 2689–2696 (2011). doi:10.1039/c1nr10124b
- B.D. Gates, Q. Xu, M. Stewart, D. Ryan, C.G. Willson, G.M. Whitesides, New approaches to nanofabrication: molding, printing, and other techniques. Chem. Rev. 105(4), 1171–1196 (2005). doi:10.1021/cr030076o
- J.C. Love, B.W. Daniel, M.W. George, Dekker Encyclopedia of Nanoscience and Nanotechnology—Six Volume Set (Print Version) (CRC Press, 2004). doi:10.1201/9781439834398.ch174
- C.J. Bettinger, K.M. Cyr, A. Matsumoto, R. Langer, J.T. Borenstein, D.L. Kaplan, Silk fibroin microfluidic devices. Adv. Mater. 19(5), 2847–2850 (2007). doi:10.1002/adma.200602487
- A. Paguirigan, D.J. Beebe, Gelatin based microfluidic devices for cell culture. Lab Chip 6(3), 407–413 (2006). doi:10.1039/b517524k
- L. Robert, J.B. Christopher, T.B. Jeffrey, in Nanotechnology and Tissue Engineering (CRC Press, 2008), pp. 87–119. doi:10.1201/9781420051834.ch4
- A. Brown, G.A. Burke, B.J. Meenan, Patterned cell culture substrates created by hot embossing of tissue culture treated polystyrene. J. Mater. Sci. Mater. Med. 24(12), 2797–2807 (2013). doi:10.1007/s10856-013-5011-5
- A.R. Jung, R.Y. Kim, H.W. Kim, K.R. Shrestha, S.H. Jeon, K.J. Cha, Y.H. Park, D.S. Kim, J.Y. Lee, Nanoengineered polystyrene surfaces with nanopore array pattern alters cytoskeleton organization and enhances induction of neural differentiation of human adipose-derived stem cells. Tissue Eng. A 21(13–14), 2115–2124 (2015). doi:10.1089/ten.tea.2014.0346
- P.M. Mendes, Cellular nanotechnology: making biological interfaces smarter. Chem. Soci. Rev. 42(24), 9207–9218 (2013). doi:10.1039/c3cs60198f
- MathSciNet
- D. Kluge, J.C. Singer, B.R. Neugirg, J.W. Neubauer, H.-W. Schmidt, A. Fery, Top–down meets bottom–up: a comparison of the mechanical properties of melt electrospun and self-assembled 1,3,5-benzenetrisamide fibers. Polymer 53(25), 5754–5759 (2012). doi:10.1016/j.polymer.2012.10.016
- G. Luo, K.S. Teh, Y. Liu, X. Zang, Z. Wen, L. Lin, Direct-write, self-aligned electrospinning on paper for controllable fabrication of three-dimensional structures. ACS Appl. Mater. Interfaces 7(50), 27765–27770 (2015). doi:10.1021/acsami.5b08909
- P.K. Chu, J.Y. Chen, L.P. Wang, N. Huang, Plasma-surface modification of biomaterials. Mater. Sci. Eng. R 36(5–6), 143–206 (2002). doi:10.1016/S0927-796X(02)00004-9
- F. Kantawong, R. Burchmore, N. Gadegaard, R.O.C. Oreffo, M.J. Dalby, Proteomic analysis of human osteoprogenitor response to disordered nanotopography. J. R. Soc. Interface 6(40), 1075–1086 (2009). doi:10.1098/rsif.2008.0447
- W.A. Loesberg, J. te Riet, F.C. van Delft, P. Schon, C.G. Figdor, S. Speller, J.J. van Loon, X.F. Walboomers, J.A. Jansen, The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 28(27), 3944–3951 (2007). doi:10.1016/j.biomaterials.2007.05.030
- K. Chung, J.A. DeQuach, K.L. Christman, Nanopatterned interfaces for controlling cell behavior. Nano LIFE 1(2), 63–77 (2010). doi:10.1142/S1793984410000055
- M. Benjamin, S. M. Gregory, in Nanotechnology and Tissue Engineering (CRC Press, 2008), pp. 261–282. doi:10.1201/9781420051834.ch10
- A.T. Ampere, Recent developments in micromilling using focused ion beam technology. J. Micromech. Microeng. 14(4), R15 (2004). doi:10.1088/0960-1317/14/4/R01
- Y. Kim, A.Y. Abuelfilat, S.P. Hoo, A. Al-Abboodi, B. Liu, T. Ng, P. Chan, J. Fu, Tuning the surface properties of hydrogel at the nanoscale with focused ion irradiation. Soft Matter 10(42), 8448–8456 (2014). doi:10.1039/C4SM01061B
- A. Minor, in Introduction to Focused Ion Beams: Instrumentation, Theory, Techniques and Practice, ed by L.A. Giannuzzi, F.A. Stevie (Springer, New York, 2005, 27(1), pp. 56–56). ISBN 038723116-1. doi:10.1002/sca.4950270109
- T. Hasebe, S. Nagashima, Y. Yoshimoto, A. Hotta, T. Suzuki, Tailoring surface topographies of polymers by using ion beam: recent advances and the potential applications in biomedical and tissue engineering. Nucl. Instrum. Methods Phys. Res., Sect. B 282, 134–136 (2012). doi:10.1016/j.nimb.2011.08.066
- M.T. Fitch, J. Silver, CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209(2), 294–301 (2008). doi:10.1016/j.expneurol.2007.05.014
- W.C. Chang, E. Hawkes, C.G. Keller, D.W. Sretavan, Axon repair: surgical application at a subcellular scale. Wiley interdisciplinary reviews. Nanomed. Nanobiotechn. 2(2), 151–161 (2010). doi:10.1002/wnan.76
- M. Lorenzoni, F. Brandi, S. Dante, A. Giugni, B. Torre, Simple and effective graphene laser processing for neuron patterning application. Sci. Rep. 3, 1954 (2013). doi:10.1038/srep01954
- N.M. Dowell-Mesfin, M.A. Abdul-Karim, A.M.P. Turner, S. Schanz, H.G. Craighead, B. Roysam, J.N. Turner, W. Shain, Topographically modified surfaces affect orientation and growth of hippocampal neurons. J. Neural Eng. 1(2), 78–90 (2004). doi:10.1088/1741-2560/1/2/003
- C. Simitzi, P. Efstathopoulos, A. Kourgiantaki, A. Ranella, I. Charalampopoulos et al., Laser fabricated discontinuous anisotropic microconical substrates as a new model scaffold to control the directionality of neuronal network outgrowth. Biomaterials 67, 115–128 (2015). doi:10.1016/j.biomaterials.2015.07.008
- S.I. Nikolaev, A.R. Gallyamov, G.V. Mamin, Y.A. Chelyshev, Poly(epsilon-caprolactone) nerve conduit and local delivery of vegf and fgf2 genes stimulate neuroregeneration. Bull. Exp. Biol. Med. 157(1), 155–158 (2014). doi:10.1007/s10517-014-2513-1
- R. Junka, X. Yu, Novel acellular scaffold made from decellularized schwann cell sheets for peripheral nerve regeneration. Regen. Eng. Transl. Med. 1(1), 22–31 (2015). doi:10.1007/s40883-015-0003-2
- R.J. McMurtrey, Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 11(6), 066009 (2014). doi:10.1088/1741-2560/11/6/066009
- D. Mozaffarian, E.J. Benjamin, A.S. Go, D.K. Arnett, M.J. Blaha et al., Heart disease and stroke statistics—2016 update: a report from the american heart association. Circulation 133(4), 447–454 (2016). doi:10.1161/CIR.0000000000000350
- N.M. Malara, V. Trunzo, G. Musolino, S. Aprigliano, G. Rotta et al., Soluble CD54 induces human endothelial cells ex vivo expansion useful for cardiovascular regeneration and tissue engineering application. IJC Heart Vasc. 6, 48–53 (2015). doi:10.1016/j.ijcha.2015.01.004
- Y.W. Chun, S.W. Crowder, S.C. Mehl, X. Wang, H. Bae, H.-J. Sung, Therapeutic application of nanotechnology in cardiovascular and pulmonary regeneration. Comput. Struct. Biotechn. J. 7(8), 1–7 (2013). doi:10.5936/csbj.201304005
- S. Pagliari, A.C. Vilela-Silva, G. Forte, F. Pagliari, C. Mandoli et al., Cooperation of biological and mechanical signals in cardiac progenitor cell differentiation. Adv. Mater. 23(4), 514–518 (2011). doi:10.1002/adma.201003479
- C. Mandoli, F. Pagliari, S. Pagliari, G. Forte, P. Di Nardo, S. Licoccia, E. Traversa, Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 20(10), 1617–1624 (2010). doi:10.1002/adfm.200902363
- P.H. Kim, J.Y. Cho, Myocardial tissue engineering using electrospun nanofiber composites. BMB Rep. 49(1), 26–36 (2016). doi:10.5483/BMBRep.2016.49.1.165
- P. Zorlutuna, P. Vadgama, V. Hasirci, Both sides nanopatterned tubular collagen scaffolds as tissue-engineered vascular grafts. J. Tissue Eng. Regen. Med. 4(8), 628–637 (2010). doi:10.1002/term.278
- D.A. Stout, J. Yoo, A.N. Santiago-Miranda, T.J. Webster, Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. Int. J. Nanomed. 7, 5653–5669 (2012). doi:10.2147/IJN.S34574
- H. Sun, S. Lu, X.X. Jiang, X. Li, H. Li, Q. Lin, Y. Mou, Y. Zhao, Y. Han, J. Zhou, C. Wang, Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the beta1-integrin-mediated signaling pathway. Biomaterials 55, 84–95 (2015). doi:10.1016/j.biomaterials.2015.03.030
- V. Martinelli, G. Cellot, F.M. Toma, C.S. Long, J.H. Caldwell et al., Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 12(4), 1831–1838 (2012). doi:10.1021/nl204064s
- T. Dvir, B.P. Timko, M.D. Brigham, S.R. Naik, S.S. Karajanagi et al., Nanowired three dimensional cardiac patches. Nat. Nanotechn. 6(11), 720–725 (2011). doi:10.1038/nnano.2011.160
- L. Susan, K.C. Casey, R. Seeram, in Nanotechnology and Regenerative Engineering (CRC Press, 2014), pp. 407–434. doi:10.1201/b17444-20
- H.N. Chia, B.M. Wu, Recent advances in 3D printing of biomaterials. J. Biol. Eng. 9, 4 (2015). doi:10.1186/s13036-015-0001-4
- Z. Karimi, M. Ghorbani, B. Hashemibeni, H. Bahramian, Evaluation of the proliferation and viability rates of nucleus pulposus cells of human intervertebral disk in fabricated chitosan-gelatin scaffolds by freeze drying and freeze gelation methods. Adv. Biomed. Res. 4, 251 (2015). doi:10.4103/2277-9175.170676
- J.M. Holzwarth, P.X. Ma, Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 32(36), 9622–9629 (2011). doi:10.1016/j.biomaterials.2011.09.009
- S.-H. Shin, O. Purevdorj, O. Castano, J.A. Planell, H.-W. Kim, A short review: recent advances in electrospinning for bone tissue regeneration. J. Tissue Eng. 3(1), 2041731412443530 (2012). doi:10.1177/2041731412443530
- K.M. Tyler, B. Morgan, S. Young-Joon, K. Hyun-Wook, L. Sang Jin, J.Y. James, A. Anthony, A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication 7(3), 035003 (2015). doi:10.1088/1758-5090/7/3/035003
- H. Seitz, W. Rieder, S. Irsen, B. Leukers, C. Tille, Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B 74(2), 782–788 (2005). doi:10.1002/jbm.b.30291
- L.H. Nguyen, N. Annabi, M. Nikkhah, H. Bae, L. Binan, S. Park, Y. Kang, Y. Yang, A. Khademhosseini, Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng. B 18(5), 363–382 (2012). doi:10.1089/ten.teb.2012.0012
- S. Camarero-Espinosa, B. Rothen-Rutishauser, E.J. Foster, C. Weder, Articular cartilage: from formation to tissue engineering. Biomater. Sci. 4, 734–767 (2016). doi:10.1039/C6BM00068A
- V.E. Santo, M.E. Gomes, J.F. Mano, R.L. Reis, From nano- to macro-scale: nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomedicine 7(7), 1045–1066 (2012). doi:10.2217/nnm.12.78
- G. Tetteh, A.S. Khan, R.M. Delaine-Smith, G.C. Reilly, I.U. Rehman, Electrospun polyurethane/hydroxyapatite bioactive Scaffolds for bone tissue engineering: The role of solvent and hydroxyapatite particles. J. Mech. Behav. Biomed. Mater. 39, 95–110 (2014). doi:10.1016/j.jmbbm.2014.06.019
- D. Yuan, Z. Chen, T. Lin, X. Luo, H. Dong, G. Feng, Cartilage tissue engineering using combination of chitosan hydrogel and mesenchymal stem cells. J. Chem. 2015, 530607 (2015). doi:10.1155/2015/530607
- B.J. Klotz, D. Gawlitta, A.J.W.P. Rosenberg, J. Malda, F.P.W. Melchels, Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechn. 34(5), 394–407 (2016). doi:10.1016/j.tibtech.2016.01.002
- W. Schuurman, P.A. Levett, M.W. Pot, P.R. van Weeren, W.J.A. Dhert et al., Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 13(5), 551–561 (2013). doi:10.1002/mabi.201200471
- K.W.M. Boere, J. Visser, H. Seyednejad, S. Rahimian, D. Gawlitta et al., Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 10(6), 2602–2611 (2014). doi:10.1016/j.actbio.2014.02.041
- W. Bian, N. Bursac, Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng. Med. Biol. Mag. 27(5), 109–113 (2008). doi:10.1109/MEMB.2008.928460
- C.A. Rossi, M. Pozzobon, P. De Coppi, Advances in musculoskeletal tissue engineering: moving towards therapy. Organogenesis 6(3), 167–172 (2010). doi:10.4161/org.6.3.12419
- D. Klumpp, R.E. Horch, U. Kneser, J.P. Beier, Engineering skeletal muscle tissue–new perspectives in vitro and in vivo. J. Cell Mol. Med. 14(11), 2622–2629 (2010). doi:10.1111/j.1582-4934.2010.01183.x
- S. Ostrovidov, V. Hosseini, S. Ahadian, T. Fujie, S.P. Parthiban et al., Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng. B 20(5), 403–436 (2014). doi:10.1089/ten.teb.2013.0534
- P.Y. Wang, H.T. Yu, W.B. Tsai, Modulation of alignment and differentiation of skeletal myoblasts by submicron ridges/grooves surface structure. Biotechnol. Bioeng. 106(2), 285–294 (2010). doi:10.1002/bit.22697
- J.Y. Shen, M.B. Chan-Park, Z.Q. Feng, V. Chan, Z.W. Feng, UV-embossed microchannel in biocompatible polymeric film: application to control of cell shape and orientation of muscle cells. J. Biomed. Mater. Res. B 77(2), 423–430 (2006). doi:10.1002/jbm.b.30449
- K.J. Aviss, J.E. Gough, S. Downes, Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cell Mater. 19(1), 193–204 (2010)
- L. Altomare, N. Gadegaard, L. Visai, M.C. Tanzi, S. Fare, Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 6(6), 1948–1957 (2010). doi:10.1016/j.actbio.2009.12.040
- M.J. Dalby, Cellular response to low adhesion nanotopographies. Int. J. Nanomed. 2(3), 373–381 (2007)
- A.S. Curtis, N. Gadegaard, M.J. Dalby, M.O. Riehle, C.D. Wilkinson, G. Aitchison, Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans. Nanobiosci. 3(1), 61–65 (2004). doi:10.1109/TNB.2004.824276
- T.J. Webster, C. Ergun, R.H. Doremus, R.W. Siegel, R. Bizios, Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 21(17), 1803–1810 (2000). doi:10.1016/S0142-9612(00)00075-2
- T.J. Webster, L.S. Schadler, R.W. Siegel, R. Bizios, Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 7(3), 291–301 (2001). doi:10.1089/10763270152044152
- R. Murugan, S. Ramakrishna, Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 13(8), 1845–1866 (2007). doi:10.1089/ten.2006.0078
- S. Jana, M. Zhang, Fabrication of 3D aligned nanofibrous tubes by direct electrospinning. J. Mater. Chem. B 1(20), 2575–2581 (2013). doi:10.1039/c3tb20197j
- E. Catalano, A. Cochis, E. Varoni, L. Rimondini, B. Azzimonti, Tissue-engineered skin substitutes: an overview. J. Artif. Organs. 16(4), 397–403 (2013). doi:10.1007/s10047-013-0734-0
- R. Gupta, D.T. Woodley, M. Chen, Epidermolysis bullosa acquisita. Clin. Dermatol. 30(1), 60–69 (2012). doi:10.1016/j.clindermatol.2011.03.011
- V.G. Shalini, N.J. Eric, S.N. Lakshmi, in Nanotechnology and Regenerative Engineering(CRC Press, 2014), pp. 343–366. doi:10.1201/b17444-17
- V. Lee, G. Singh, J.P. Trasatti, C. Bjornsson, X. Xu, T.N. Tran, S.-S. Yoo, G. Dai, P. Karande, Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. C 20(6), 473–484 (2014). doi:10.1089/ten.tec.2013.0335
- R.E. Billingham, J. Reynolds, Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br. J. Plast. Surg. 5(1), 25–36 (1952). doi:10.1016/S0007-1226(52)80004-9
- M.F. Bin Mh Busra, S.R. Chowdhury, F. Bin Ismail, A. Bin Saim, R.H. Idrus, Tissue-engineered skin substitute enhances wound healing after radiation therapy. Adv. Skin Wound Care 29(3), 120–129 (2016). doi:10.1097/01.ASW.0000480556.78111.e4
- J. Kober, A. Gugerell, M. Schmid, L.P. Kamolz, M. Keck, Generation of a fibrin based three-layered skin substitute. Biomed. Res. Int. 2015, 170427 (2015). doi:10.1155/2015/170427
- F. Wang, M. Wang, Z. She, K. Fan, C. Xu, B. Chu, C. Chen, S. Shi, R. Tan, Collagen/chitosan based two-compartment and bi-functional dermal scaffolds for skin regeneration. Mater. Sci. Eng. C 52, 155–162 (2015). doi:10.1016/j.msec.2015.03.013
- Y. Matsumoto, K. Ikeda, Y. Yamaya, K. Yamashita, T. Saito et al., The usefulness of the collagen and elastin sponge derived from salmon as an artificial dermis and scaffold for tissue engineering. Biomed. Res. 32(1), 29–36 (2011). doi:10.2220/biomedres.32.29
- I.P. Monteiro, D. Gabriel, B.P. Timko, M. Hashimoto, S. Karajanagi, R. Tong, A.P. Marques, R.L. Reis, D.S. Kohane, A two-component pre-seeded dermal-epidermal scaffold. Acta Biomater. 10(12), 4928–4938 (2014). doi:10.1016/j.actbio.2014.08.029
- S. Rastogi, M. Modi, B. Sathian, The efficacy of collagen membrane as a biodegradable wound dressing material for surgical defects of oral mucosa: a prospective study. J. Oral Maxillofac. Surg. 67(8), 1600–1606 (2009). doi:10.1016/j.joms.2008.12.020
- Z. Ghanavati, N. Neisi, V. Bayati, M. Makvandi, The influence of substrate topography and biomaterial substance on skin wound healing. Anat. Cell Biol. 48(4), 251–257 (2015). doi:10.5115/acb.2015.48.4.251
- P.T. Hwang, K. Murdock, G.C. Alexander, A.D. Salaam, J.I. Ng, D.J. Lim, D. Dean, H.W. Jun, Poly(epsilon-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. A 104(4), 1017–1029 (2016). doi:10.1002/jbm.a.35614
References
J. Hwang, Y. Jeong, J.M. Park, K.H. Lee, J.W. Hong, J. Choi, Biomimetics: forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 8(10), 5701–5713 (2015). doi:10.2147/IJN.S83642
M.J. Webber, E.A. Appel, E.W. Meijer, R. Langer, Supramolecular biomaterials. Nat. Mater. 15(1), 13–26 (2015). doi:10.1038/nmat4474
T.U. Luu, S.C. Gott, B.W. Woo, M.P. Rao, W.F. Liu, Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl. Mater. Interfaces 7(51), 28665–28672 (2015). doi:10.1021/acsami.5b10589
N. Mauro, A. Manfredi, E. Ranucci, P. Procacci, M. Laus, D. Antonioli, C. Mantovani, V. Magnaghi, P. Ferruti, Degradable poly(amidoamine) hydrogels as scaffolds for in vitro culturing of peripheral nervous system cells. Macromol. Biosci. 13(3), 332–347 (2013). doi:10.1002/mabi.201200354
W. Zhu, C. O’Brien, J.R. O’Brien, L.G. Zhang, 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine 9(6), 859–875 (2014). doi:10.2217/nnm.14.36
P. Haisheng, L. Xunpei, W. Ran, J. Feng, D. Liang, W. Qun, Emerging nanostructured materials for musculoskeletal tissue engineering. J. Mater. Chem. B 2(38), 6435–6461 (2014). doi:10.1039/c4tb00344f
H. Park, C. Cannizzaro, G. Vunjak-Novakovic, R. Langer, C.A. Vacanti, O.C. Farokhzad, Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 13(8), 1867–1877 (2007). doi:10.1089/ten.2006.0198
B.N. Brown, B.D. Ratner, S.B. Goodman, S. Amar, S.F. Badylak, Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33(15), 3792–3802 (2012). doi:10.1016/j.biomaterials.2012.02.034
B.D. Ratner, S.J. Bryant, Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 6, 41–75 (2004). doi:10.1146/annurev.bioeng.6.040803.140027
M. Veiseh, A. Nikjoo, E.A. Turley, M.J. Bissell, Nanotechnology and regenerative engineering: the scaffold: from microenvironment to nanoenvironment: ultrastructure and function of extracellular matrix, ed. by C.T. Laurencin, L.S. Nair (CRC Press, Boca Raton, 2015), pp 39–62
F. Gattazzo, A. Urciuolo, P. Bonaldo, Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 1840(8), 2506–2519 (2014). doi:10.1016/j.bbagen.2014.01.010
R.G. Flemming, C.J. Murphy, G.A. Abrams, S.L. Goodman, P.F. Nealey, Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 20(6), 573–588 (1999)
F. Gentile, R. La Rocca, G. Marinaro, A. Nicastri, A. Toma et al., Differential cell adhesion on mesoporous silicon substrates. ACS Appl. Mater. Interfaces 4(6), 2903–2911 (2012). doi:10.1021/am300519a
F. Gentile, L. Tirinato, E. Battista, F. Causa, C. Liberale, E.M. di Fabrizio, P. Decuzzi, Cells preferentially grow on rough substrates. Biomaterials 31(28), 7205–7212 (2010). doi:10.1016/j.biomaterials.2010.06.016
T. Limongi, F. Cesca, F. Gentile, R. Marotta, R. Ruffilli et al., Nanostructured superhydrophobic substrates trigger the development of 3d neuronal networks. Small 9(3), 402–412 (2013). doi:10.1002/smll.201201377
F. Cesca, T. Limongi, A. Accardo, A. Rocchi, M. Orlando, V. Shalabaeva, E. Di Fabrizio, F. Benfenati, Fabrication of biocompatible free-standing nanopatterned films for primary neuronal cultures. RSC Adv. 4(86), 45696–45702 (2014). doi:10.1039/c4ra08361j
T. Limongi, R. Schipani, A. Di Vito, A. Giugni, M. Francardi et al., Photolithography and micromolding techniques for the realization of 3D polycaprolactone scaffolds for tissue engineering applications. Microelectron. Eng. 141, 135–139 (2015). doi:10.1016/j.mee.2015.02.030
T. Limongi, E. Miele, V. Shalabaeva, R. La Rocca, R. Schipani et al., Development, characterization and cell cultural response of 3d biocompatible micro-patterned poly-ε-caprolactone scaffolds designed and fabricated integrating lithography and micromolding fabrication techniques. J. Tissue Sci. Eng. 6(1), 5 (2015). doi:10.4172/2157-7552.1000145
K.M. Ainslie, T.A. Desai, Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip 8(11), 1864–1878 (2008). doi:10.1039/b806446f
C.W. Park, Y.S. Rhee, S.H. Park, S.D. Danh, S.H. Ahn, S.C. Chi, E.S. Park, In vitro/in vivo evaluation of NCDS-micro-fabricated biodegradable implant. Arch. Pharm. Res. 33(3), 427–432 (2010). doi:10.1007/s12272-010-0312-4
A.S. Curtis, C.D. Wilkinson, J. Crossan, C. Broadley, H. Darmani, K.K. Johal, H. Jorgensen, W. Monaghan, An in vivo microfabricated scaffold for tendon repair. Eur. Cell Mater. 9, 50–57 (2005)
D. Shi, X. Xu, Y. Ye, K. Song, Y. Cheng, J. Di, Q. Hu, J. Li, H. Ju, Q. Jiang, Z. Gu, Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 10(1), 1292–1299 (2016). doi:10.1021/acsnano.5b06663
K. Baranes, M. Shevach, O. Shefi, T. Dvir, Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 16(5), 2916–2920 (2015). doi:10.1021/acs.nanolett.5b04033
E.L. Hopley, S. Salmasi, D.M. Kalaskar, A.M. Seifalian, Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 32(5), 1000–1014 (2014). doi:10.1016/j.biotechadv.2014.05.003
A. Childs, U.D. Hemraz, N.J. Castro, H. Fenniri, L.G. Zhang, Novel biologically-inspired rosette nanotube PLLA scaffolds for improving human mesenchymal stem cell chondrogenic differentiation. Biomed. Mater. 8(6), 065003 (2013). doi:10.1088/1748-6041/8/6/065003
Y.C. Shin, J.H. Lee, M.J. Kim, S.W. Hong, B. Kim, J.K. Hyun, Y.S. Choi, J.C. Park, D.W. Han, Stimulating effect of graphene oxide on myogenesis of C2C12 myoblasts on RGD peptide-decorated PLGA nanofiber matrices. J. Biol. Eng. 9, 22 (2015). doi:10.1186/s13036-015-0020-1
A. Raspa, A. Marchini, R. Pugliese, M. Mauri, M. Maleki, R. Vasita, F. Gelain, A biocompatibility study of new nanofibrous scaffolds for nervous system regeneration. Nanoscale 8(1), 253–265 (2015). doi:10.1039/C5NR03698D
J.M. Szymanski, Q. Jallerat, A.W. Feinberg, ECM protein nanofibers and nanostructures engineered using surface-initiated assembly. J. Vis. Exp. 86, e51176 (2014). doi:10.3791/51176
C. Mota, S. Danti, D. D’Alessandro, L. Trombi, C. Ricci et al., Multiscale fabrication of biomimetic scaffolds for tympanic membrane tissue engineering. Biofabrication 7(2), 025005 (2015). doi:10.1088/1758-5090/7/2/025005
E. Zanchetta, E. Guidi, G. Della Giustina, M. Sorgato, M. Krampera et al., Injection molded polymeric micropatterns for bone regeneration study. ACS Appl. Mater. Interfaces 7(13), 7273–7281 (2015). doi:10.1021/acsami.5b00481
R.E. McMahon, X. Qu, A.C. Jimenez-Vergara, C.A. Bashur, S.A. Guelcher, A.S. Goldstein, M.S. Hahn, Hydrogel-electrospun mesh composites for coronary artery bypass grafts. Tissue Eng. C 17(4), 451–461 (2011). doi:10.1089/ten.tec.2010.0427
M.J. Lima, V.M. Correlo, R.L. Reis, Micro/nano replication and 3D assembling techniques for scaffold fabrication. Mater. Sci. Eng. Part C 42, 615–621 (2014). doi:10.1016/j.msec.2014.05.064
V. Raffa, O. Vittorio, V. Pensabene, A. Menciassi, P. Dario, FIB-nanostructured surfaces and investigation of Bio/nonbio interactions at the nanoscale. IEEE Trans. Nanobiosci. 7(1), 1–10 (2008). doi:10.1109/TNB.2008.2000143
F. De Angelis, C. Liberale, M.L. Coluccio, G. Cojoc, E. Di Fabrizio, Emerging fabrication techniques for 3D nano-structuring in plasmonics and single molecule studies. Nanoscale 3(7), 2689–2696 (2011). doi:10.1039/c1nr10124b
B.D. Gates, Q. Xu, M. Stewart, D. Ryan, C.G. Willson, G.M. Whitesides, New approaches to nanofabrication: molding, printing, and other techniques. Chem. Rev. 105(4), 1171–1196 (2005). doi:10.1021/cr030076o
J.C. Love, B.W. Daniel, M.W. George, Dekker Encyclopedia of Nanoscience and Nanotechnology—Six Volume Set (Print Version) (CRC Press, 2004). doi:10.1201/9781439834398.ch174
C.J. Bettinger, K.M. Cyr, A. Matsumoto, R. Langer, J.T. Borenstein, D.L. Kaplan, Silk fibroin microfluidic devices. Adv. Mater. 19(5), 2847–2850 (2007). doi:10.1002/adma.200602487
A. Paguirigan, D.J. Beebe, Gelatin based microfluidic devices for cell culture. Lab Chip 6(3), 407–413 (2006). doi:10.1039/b517524k
L. Robert, J.B. Christopher, T.B. Jeffrey, in Nanotechnology and Tissue Engineering (CRC Press, 2008), pp. 87–119. doi:10.1201/9781420051834.ch4
A. Brown, G.A. Burke, B.J. Meenan, Patterned cell culture substrates created by hot embossing of tissue culture treated polystyrene. J. Mater. Sci. Mater. Med. 24(12), 2797–2807 (2013). doi:10.1007/s10856-013-5011-5
A.R. Jung, R.Y. Kim, H.W. Kim, K.R. Shrestha, S.H. Jeon, K.J. Cha, Y.H. Park, D.S. Kim, J.Y. Lee, Nanoengineered polystyrene surfaces with nanopore array pattern alters cytoskeleton organization and enhances induction of neural differentiation of human adipose-derived stem cells. Tissue Eng. A 21(13–14), 2115–2124 (2015). doi:10.1089/ten.tea.2014.0346
P.M. Mendes, Cellular nanotechnology: making biological interfaces smarter. Chem. Soci. Rev. 42(24), 9207–9218 (2013). doi:10.1039/c3cs60198f
MathSciNet
D. Kluge, J.C. Singer, B.R. Neugirg, J.W. Neubauer, H.-W. Schmidt, A. Fery, Top–down meets bottom–up: a comparison of the mechanical properties of melt electrospun and self-assembled 1,3,5-benzenetrisamide fibers. Polymer 53(25), 5754–5759 (2012). doi:10.1016/j.polymer.2012.10.016
G. Luo, K.S. Teh, Y. Liu, X. Zang, Z. Wen, L. Lin, Direct-write, self-aligned electrospinning on paper for controllable fabrication of three-dimensional structures. ACS Appl. Mater. Interfaces 7(50), 27765–27770 (2015). doi:10.1021/acsami.5b08909
P.K. Chu, J.Y. Chen, L.P. Wang, N. Huang, Plasma-surface modification of biomaterials. Mater. Sci. Eng. R 36(5–6), 143–206 (2002). doi:10.1016/S0927-796X(02)00004-9
F. Kantawong, R. Burchmore, N. Gadegaard, R.O.C. Oreffo, M.J. Dalby, Proteomic analysis of human osteoprogenitor response to disordered nanotopography. J. R. Soc. Interface 6(40), 1075–1086 (2009). doi:10.1098/rsif.2008.0447
W.A. Loesberg, J. te Riet, F.C. van Delft, P. Schon, C.G. Figdor, S. Speller, J.J. van Loon, X.F. Walboomers, J.A. Jansen, The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 28(27), 3944–3951 (2007). doi:10.1016/j.biomaterials.2007.05.030
K. Chung, J.A. DeQuach, K.L. Christman, Nanopatterned interfaces for controlling cell behavior. Nano LIFE 1(2), 63–77 (2010). doi:10.1142/S1793984410000055
M. Benjamin, S. M. Gregory, in Nanotechnology and Tissue Engineering (CRC Press, 2008), pp. 261–282. doi:10.1201/9781420051834.ch10
A.T. Ampere, Recent developments in micromilling using focused ion beam technology. J. Micromech. Microeng. 14(4), R15 (2004). doi:10.1088/0960-1317/14/4/R01
Y. Kim, A.Y. Abuelfilat, S.P. Hoo, A. Al-Abboodi, B. Liu, T. Ng, P. Chan, J. Fu, Tuning the surface properties of hydrogel at the nanoscale with focused ion irradiation. Soft Matter 10(42), 8448–8456 (2014). doi:10.1039/C4SM01061B
A. Minor, in Introduction to Focused Ion Beams: Instrumentation, Theory, Techniques and Practice, ed by L.A. Giannuzzi, F.A. Stevie (Springer, New York, 2005, 27(1), pp. 56–56). ISBN 038723116-1. doi:10.1002/sca.4950270109
T. Hasebe, S. Nagashima, Y. Yoshimoto, A. Hotta, T. Suzuki, Tailoring surface topographies of polymers by using ion beam: recent advances and the potential applications in biomedical and tissue engineering. Nucl. Instrum. Methods Phys. Res., Sect. B 282, 134–136 (2012). doi:10.1016/j.nimb.2011.08.066
M.T. Fitch, J. Silver, CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209(2), 294–301 (2008). doi:10.1016/j.expneurol.2007.05.014
W.C. Chang, E. Hawkes, C.G. Keller, D.W. Sretavan, Axon repair: surgical application at a subcellular scale. Wiley interdisciplinary reviews. Nanomed. Nanobiotechn. 2(2), 151–161 (2010). doi:10.1002/wnan.76
M. Lorenzoni, F. Brandi, S. Dante, A. Giugni, B. Torre, Simple and effective graphene laser processing for neuron patterning application. Sci. Rep. 3, 1954 (2013). doi:10.1038/srep01954
N.M. Dowell-Mesfin, M.A. Abdul-Karim, A.M.P. Turner, S. Schanz, H.G. Craighead, B. Roysam, J.N. Turner, W. Shain, Topographically modified surfaces affect orientation and growth of hippocampal neurons. J. Neural Eng. 1(2), 78–90 (2004). doi:10.1088/1741-2560/1/2/003
C. Simitzi, P. Efstathopoulos, A. Kourgiantaki, A. Ranella, I. Charalampopoulos et al., Laser fabricated discontinuous anisotropic microconical substrates as a new model scaffold to control the directionality of neuronal network outgrowth. Biomaterials 67, 115–128 (2015). doi:10.1016/j.biomaterials.2015.07.008
S.I. Nikolaev, A.R. Gallyamov, G.V. Mamin, Y.A. Chelyshev, Poly(epsilon-caprolactone) nerve conduit and local delivery of vegf and fgf2 genes stimulate neuroregeneration. Bull. Exp. Biol. Med. 157(1), 155–158 (2014). doi:10.1007/s10517-014-2513-1
R. Junka, X. Yu, Novel acellular scaffold made from decellularized schwann cell sheets for peripheral nerve regeneration. Regen. Eng. Transl. Med. 1(1), 22–31 (2015). doi:10.1007/s40883-015-0003-2
R.J. McMurtrey, Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 11(6), 066009 (2014). doi:10.1088/1741-2560/11/6/066009
D. Mozaffarian, E.J. Benjamin, A.S. Go, D.K. Arnett, M.J. Blaha et al., Heart disease and stroke statistics—2016 update: a report from the american heart association. Circulation 133(4), 447–454 (2016). doi:10.1161/CIR.0000000000000350
N.M. Malara, V. Trunzo, G. Musolino, S. Aprigliano, G. Rotta et al., Soluble CD54 induces human endothelial cells ex vivo expansion useful for cardiovascular regeneration and tissue engineering application. IJC Heart Vasc. 6, 48–53 (2015). doi:10.1016/j.ijcha.2015.01.004
Y.W. Chun, S.W. Crowder, S.C. Mehl, X. Wang, H. Bae, H.-J. Sung, Therapeutic application of nanotechnology in cardiovascular and pulmonary regeneration. Comput. Struct. Biotechn. J. 7(8), 1–7 (2013). doi:10.5936/csbj.201304005
S. Pagliari, A.C. Vilela-Silva, G. Forte, F. Pagliari, C. Mandoli et al., Cooperation of biological and mechanical signals in cardiac progenitor cell differentiation. Adv. Mater. 23(4), 514–518 (2011). doi:10.1002/adma.201003479
C. Mandoli, F. Pagliari, S. Pagliari, G. Forte, P. Di Nardo, S. Licoccia, E. Traversa, Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 20(10), 1617–1624 (2010). doi:10.1002/adfm.200902363
P.H. Kim, J.Y. Cho, Myocardial tissue engineering using electrospun nanofiber composites. BMB Rep. 49(1), 26–36 (2016). doi:10.5483/BMBRep.2016.49.1.165
P. Zorlutuna, P. Vadgama, V. Hasirci, Both sides nanopatterned tubular collagen scaffolds as tissue-engineered vascular grafts. J. Tissue Eng. Regen. Med. 4(8), 628–637 (2010). doi:10.1002/term.278
D.A. Stout, J. Yoo, A.N. Santiago-Miranda, T.J. Webster, Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. Int. J. Nanomed. 7, 5653–5669 (2012). doi:10.2147/IJN.S34574
H. Sun, S. Lu, X.X. Jiang, X. Li, H. Li, Q. Lin, Y. Mou, Y. Zhao, Y. Han, J. Zhou, C. Wang, Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the beta1-integrin-mediated signaling pathway. Biomaterials 55, 84–95 (2015). doi:10.1016/j.biomaterials.2015.03.030
V. Martinelli, G. Cellot, F.M. Toma, C.S. Long, J.H. Caldwell et al., Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 12(4), 1831–1838 (2012). doi:10.1021/nl204064s
T. Dvir, B.P. Timko, M.D. Brigham, S.R. Naik, S.S. Karajanagi et al., Nanowired three dimensional cardiac patches. Nat. Nanotechn. 6(11), 720–725 (2011). doi:10.1038/nnano.2011.160
L. Susan, K.C. Casey, R. Seeram, in Nanotechnology and Regenerative Engineering (CRC Press, 2014), pp. 407–434. doi:10.1201/b17444-20
H.N. Chia, B.M. Wu, Recent advances in 3D printing of biomaterials. J. Biol. Eng. 9, 4 (2015). doi:10.1186/s13036-015-0001-4
Z. Karimi, M. Ghorbani, B. Hashemibeni, H. Bahramian, Evaluation of the proliferation and viability rates of nucleus pulposus cells of human intervertebral disk in fabricated chitosan-gelatin scaffolds by freeze drying and freeze gelation methods. Adv. Biomed. Res. 4, 251 (2015). doi:10.4103/2277-9175.170676
J.M. Holzwarth, P.X. Ma, Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 32(36), 9622–9629 (2011). doi:10.1016/j.biomaterials.2011.09.009
S.-H. Shin, O. Purevdorj, O. Castano, J.A. Planell, H.-W. Kim, A short review: recent advances in electrospinning for bone tissue regeneration. J. Tissue Eng. 3(1), 2041731412443530 (2012). doi:10.1177/2041731412443530
K.M. Tyler, B. Morgan, S. Young-Joon, K. Hyun-Wook, L. Sang Jin, J.Y. James, A. Anthony, A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication 7(3), 035003 (2015). doi:10.1088/1758-5090/7/3/035003
H. Seitz, W. Rieder, S. Irsen, B. Leukers, C. Tille, Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B 74(2), 782–788 (2005). doi:10.1002/jbm.b.30291
L.H. Nguyen, N. Annabi, M. Nikkhah, H. Bae, L. Binan, S. Park, Y. Kang, Y. Yang, A. Khademhosseini, Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng. B 18(5), 363–382 (2012). doi:10.1089/ten.teb.2012.0012
S. Camarero-Espinosa, B. Rothen-Rutishauser, E.J. Foster, C. Weder, Articular cartilage: from formation to tissue engineering. Biomater. Sci. 4, 734–767 (2016). doi:10.1039/C6BM00068A
V.E. Santo, M.E. Gomes, J.F. Mano, R.L. Reis, From nano- to macro-scale: nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomedicine 7(7), 1045–1066 (2012). doi:10.2217/nnm.12.78
G. Tetteh, A.S. Khan, R.M. Delaine-Smith, G.C. Reilly, I.U. Rehman, Electrospun polyurethane/hydroxyapatite bioactive Scaffolds for bone tissue engineering: The role of solvent and hydroxyapatite particles. J. Mech. Behav. Biomed. Mater. 39, 95–110 (2014). doi:10.1016/j.jmbbm.2014.06.019
D. Yuan, Z. Chen, T. Lin, X. Luo, H. Dong, G. Feng, Cartilage tissue engineering using combination of chitosan hydrogel and mesenchymal stem cells. J. Chem. 2015, 530607 (2015). doi:10.1155/2015/530607
B.J. Klotz, D. Gawlitta, A.J.W.P. Rosenberg, J. Malda, F.P.W. Melchels, Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechn. 34(5), 394–407 (2016). doi:10.1016/j.tibtech.2016.01.002
W. Schuurman, P.A. Levett, M.W. Pot, P.R. van Weeren, W.J.A. Dhert et al., Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 13(5), 551–561 (2013). doi:10.1002/mabi.201200471
K.W.M. Boere, J. Visser, H. Seyednejad, S. Rahimian, D. Gawlitta et al., Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 10(6), 2602–2611 (2014). doi:10.1016/j.actbio.2014.02.041
W. Bian, N. Bursac, Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng. Med. Biol. Mag. 27(5), 109–113 (2008). doi:10.1109/MEMB.2008.928460
C.A. Rossi, M. Pozzobon, P. De Coppi, Advances in musculoskeletal tissue engineering: moving towards therapy. Organogenesis 6(3), 167–172 (2010). doi:10.4161/org.6.3.12419
D. Klumpp, R.E. Horch, U. Kneser, J.P. Beier, Engineering skeletal muscle tissue–new perspectives in vitro and in vivo. J. Cell Mol. Med. 14(11), 2622–2629 (2010). doi:10.1111/j.1582-4934.2010.01183.x
S. Ostrovidov, V. Hosseini, S. Ahadian, T. Fujie, S.P. Parthiban et al., Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng. B 20(5), 403–436 (2014). doi:10.1089/ten.teb.2013.0534
P.Y. Wang, H.T. Yu, W.B. Tsai, Modulation of alignment and differentiation of skeletal myoblasts by submicron ridges/grooves surface structure. Biotechnol. Bioeng. 106(2), 285–294 (2010). doi:10.1002/bit.22697
J.Y. Shen, M.B. Chan-Park, Z.Q. Feng, V. Chan, Z.W. Feng, UV-embossed microchannel in biocompatible polymeric film: application to control of cell shape and orientation of muscle cells. J. Biomed. Mater. Res. B 77(2), 423–430 (2006). doi:10.1002/jbm.b.30449
K.J. Aviss, J.E. Gough, S. Downes, Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cell Mater. 19(1), 193–204 (2010)
L. Altomare, N. Gadegaard, L. Visai, M.C. Tanzi, S. Fare, Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 6(6), 1948–1957 (2010). doi:10.1016/j.actbio.2009.12.040
M.J. Dalby, Cellular response to low adhesion nanotopographies. Int. J. Nanomed. 2(3), 373–381 (2007)
A.S. Curtis, N. Gadegaard, M.J. Dalby, M.O. Riehle, C.D. Wilkinson, G. Aitchison, Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans. Nanobiosci. 3(1), 61–65 (2004). doi:10.1109/TNB.2004.824276
T.J. Webster, C. Ergun, R.H. Doremus, R.W. Siegel, R. Bizios, Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 21(17), 1803–1810 (2000). doi:10.1016/S0142-9612(00)00075-2
T.J. Webster, L.S. Schadler, R.W. Siegel, R. Bizios, Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 7(3), 291–301 (2001). doi:10.1089/10763270152044152
R. Murugan, S. Ramakrishna, Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 13(8), 1845–1866 (2007). doi:10.1089/ten.2006.0078
S. Jana, M. Zhang, Fabrication of 3D aligned nanofibrous tubes by direct electrospinning. J. Mater. Chem. B 1(20), 2575–2581 (2013). doi:10.1039/c3tb20197j
E. Catalano, A. Cochis, E. Varoni, L. Rimondini, B. Azzimonti, Tissue-engineered skin substitutes: an overview. J. Artif. Organs. 16(4), 397–403 (2013). doi:10.1007/s10047-013-0734-0
R. Gupta, D.T. Woodley, M. Chen, Epidermolysis bullosa acquisita. Clin. Dermatol. 30(1), 60–69 (2012). doi:10.1016/j.clindermatol.2011.03.011
V.G. Shalini, N.J. Eric, S.N. Lakshmi, in Nanotechnology and Regenerative Engineering(CRC Press, 2014), pp. 343–366. doi:10.1201/b17444-17
V. Lee, G. Singh, J.P. Trasatti, C. Bjornsson, X. Xu, T.N. Tran, S.-S. Yoo, G. Dai, P. Karande, Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. C 20(6), 473–484 (2014). doi:10.1089/ten.tec.2013.0335
R.E. Billingham, J. Reynolds, Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br. J. Plast. Surg. 5(1), 25–36 (1952). doi:10.1016/S0007-1226(52)80004-9
M.F. Bin Mh Busra, S.R. Chowdhury, F. Bin Ismail, A. Bin Saim, R.H. Idrus, Tissue-engineered skin substitute enhances wound healing after radiation therapy. Adv. Skin Wound Care 29(3), 120–129 (2016). doi:10.1097/01.ASW.0000480556.78111.e4
J. Kober, A. Gugerell, M. Schmid, L.P. Kamolz, M. Keck, Generation of a fibrin based three-layered skin substitute. Biomed. Res. Int. 2015, 170427 (2015). doi:10.1155/2015/170427
F. Wang, M. Wang, Z. She, K. Fan, C. Xu, B. Chu, C. Chen, S. Shi, R. Tan, Collagen/chitosan based two-compartment and bi-functional dermal scaffolds for skin regeneration. Mater. Sci. Eng. C 52, 155–162 (2015). doi:10.1016/j.msec.2015.03.013
Y. Matsumoto, K. Ikeda, Y. Yamaya, K. Yamashita, T. Saito et al., The usefulness of the collagen and elastin sponge derived from salmon as an artificial dermis and scaffold for tissue engineering. Biomed. Res. 32(1), 29–36 (2011). doi:10.2220/biomedres.32.29
I.P. Monteiro, D. Gabriel, B.P. Timko, M. Hashimoto, S. Karajanagi, R. Tong, A.P. Marques, R.L. Reis, D.S. Kohane, A two-component pre-seeded dermal-epidermal scaffold. Acta Biomater. 10(12), 4928–4938 (2014). doi:10.1016/j.actbio.2014.08.029
S. Rastogi, M. Modi, B. Sathian, The efficacy of collagen membrane as a biodegradable wound dressing material for surgical defects of oral mucosa: a prospective study. J. Oral Maxillofac. Surg. 67(8), 1600–1606 (2009). doi:10.1016/j.joms.2008.12.020
Z. Ghanavati, N. Neisi, V. Bayati, M. Makvandi, The influence of substrate topography and biomaterial substance on skin wound healing. Anat. Cell Biol. 48(4), 251–257 (2015). doi:10.5115/acb.2015.48.4.251
P.T. Hwang, K. Murdock, G.C. Alexander, A.D. Salaam, J.I. Ng, D.J. Lim, D. Dean, H.W. Jun, Poly(epsilon-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. A 104(4), 1017–1029 (2016). doi:10.1002/jbm.a.35614