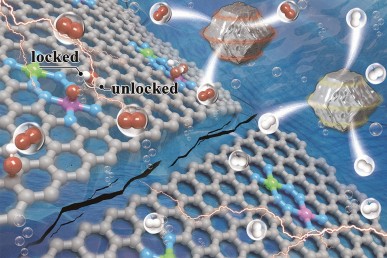
Atomically Dispersed Dual-Metal Sites Showing Unique Reactivity and Dynamism for Electrocatalysis
Corresponding Author: Jie‑Peng Zhang
Nano-Micro Letters,
Vol. 15 (2023), Article Number: 120
Abstract
The real structure and in situ evolution of catalysts under working conditions are of paramount importance, especially for bifunctional electrocatalysis. Here, we report asymmetric structural evolution and dynamic hydrogen-bonding promotion mechanism of an atomically dispersed electrocatalyst. Pyrolysis of Co/Ni-doped MAF-4/ZIF-8 yielded nitrogen-doped porous carbons functionalized by atomically dispersed Co–Ni dual-metal sites with an unprecedented N8V4 structure, which can serve as an efficient bifunctional electrocatalyst for overall water splitting. More importantly, the electrocatalyst showed remarkable activation behavior due to the in situ oxidation of the carbon substrate to form C–OH groups. Density functional theory calculations suggested that the flexible C–OH groups can form reversible hydrogen bonds with the oxygen evolution reaction intermediates, giving a bridge between elementary reactions to break the conventional scaling relationship.
Highlights:
1 An atomically dispersed catalyst with unprecedented N8V4 Co-Ni dual-metal sites is synthesized, which shows interesting asymmetric in situ structural evolution and serves as a quasi-bifunctional catalyst for water splitting.
2 The flexible C–OH groups generated by in situ oxidation can reversibly turn on/off the hydrogen-bonding interaction with the oxygen evolution reaction intermediates to break the conventional scaling relationship.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Y. Jiao, Y. Zheng, M. Jaroniec, S.Z. Qiao, Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015). https://doi.org/10.1039/c4cs00470a
- J. Wang, Y. Gao, H. Kong, J. Kim, S. Choi et al., Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 49, 9154–9196 (2020). https://doi.org/10.1039/d0cs00575d
- H.-Y. Wang, M.-L. Sun, J.-T. Ren, Z.-Y. Yuan, Circumventing challenges: design of anodic electrocatalysts for hybrid water electrolysis systems. Adv. Energy Mater. 13, 2203568 (2021). https://doi.org/10.1002/aenm.202203568
- H. Fan, Y. Wang, F. Gao, L. Yang, M. Liu et al., Hierarchical sulfur and nitrogen co-doped carbon nanocages as efficient bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. J. Energy Chem. 34, 64–71 (2019). https://doi.org/10.1016/j.jechem.2018.09.003
- X. Tian, P. Zhao, W. Sheng, Hydrogen evolution and oxidation: mechanistic studies and material advances. Adv. Mater. 31, 1808066 (2019). https://doi.org/10.1002/adma.201808066
- S. Park, Y. Shao, J. Liu, Y. Wang, Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: status and perspective. Energy Environ. Sci. 5, 9331–9344 (2012). https://doi.org/10.1039/c2ee22554a
- M. Yu, E. Budiyanto, H. Tüysüz, Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 61, 202103824 (2022). https://doi.org/10.1002/anie.202103824
- J.-X. Wu, C.-T. He, G.-R. Li, J.-P. Zhang, An inorganic-MOF-inorganic approach to ultrathin CuO decorated Cu-C hybrid nanorod arrays for an efficient oxygen evolution reaction. J. Mater. Chem. A 6, 19176–19181 (2018). https://doi.org/10.1039/c8ta06069j
- J.-T. Ren, Y. Yao, Z.-Y. Yuan, Fabrication strategies of porous precious-metal-free bifunctional electrocatalysts for overall water splitting: recent advances. Green Energy Environ. 6, 620–643 (2021). https://doi.org/10.1016/j.gee.2020.11.023
- J.-X. Wu, P.P. Bag, Y.-T. Xu, L. Gong, C.-T. He et al., Graphene-like hydrogen-bonded melamine-cyanuric acid supramolecular nanosheets as pseudo-porous catalyst support. Adv. Mater. 33, 2007368 (2021). https://doi.org/10.1002/adma.202007368
- Y. Zhang, M. Shi, C. Wang, Y. Zhu, N. Li et al., Vertically aligned NiS2/CoS2/MoS2 nanosheet array as an efficient and low-cost electrocatalyst for hydrogen evolution reaction in alkaline media. Sci. Bull. 65, 359–366 (2020). https://doi.org/10.1016/j.scib.2019.12.003
- Y. Yin, Y. Zhang, T. Gao, T. Yao, X. Zhang et al., Synergistic phase and disorder engineering in 1T-MoSe2 nanosheets for enhanced hydrogen-evolution reaction. Adv. Mater. 29, 1700311 (2017). https://doi.org/10.1002/adma.201700311
- W.-F. Chen, K. Sasaki, C. Ma, A.I. Frenkel, N. Marinkovic et al., Hydrogen-evolution catalysts based on non-noble metal nickel-molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012). https://doi.org/10.1002/anie.201200699
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu et al., Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140, 7748–7759 (2018). https://doi.org/10.1021/jacs.8b04546
- F. Dionigi, Z. Zeng, I. Sinev, T. Merzdorf, S. Deshpande et al., In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 11, 2522 (2020). https://doi.org/10.1038/s41467-020-16237-1
- J.Y. Chen, L. Dang, H. Liang, W. Bi, J.B. Gerken et al., Operando analysis of NiFe and Fe oxyhydroxide electrocatalysts for water oxidation: detection of Fe4+ by Mössbauer spectroscopy. J. Am. Chem. Soc. 137, 15090–15093 (2015). https://doi.org/10.1021/jacs.5b10699
- Y. Jia, L. Zhang, G. Gao, H. Chen, B. Wang et al., A heterostructure coupling of exfoliated Ni–Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 29, 1700017 (2017). https://doi.org/10.1002/adma.201700017
- H. Wang, Z.N. Chen, D. Wu, M. Cao, F. Sun et al., Significantly enhanced overall water splitting performance by partial oxidation of Ir through Au modification in core-shell alloy structure. J. Am. Chem. Soc. 143, 4639–4645 (2021). https://doi.org/10.1021/jacs.0c12740
- L. Sun, Q. Luo, Z. Dai, F. Ma, Material libraries for electrocatalytic overall water splitting. Coordin. Chem. Rev. 444, 214049 (2021). https://doi.org/10.1016/j.ccr.2021.214049
- L. Chen, J.-T. Ren, Z.-Y. Yuan, Design strategies of phosphorus-containing catalysts for photocatalytic, photoelectrochemical and electrocatalytic water splittings. Green Chem. 24, 713–747 (2022). https://doi.org/10.1039/d1gc03768d
- B. Guo, Y. Ding, H. Huo, X. Wen, X. Ren et al., Recent advances of transition metal basic salts for electrocatalytic oxygen evolution reaction and overall water electrolysis. Nano Micro Lett. 15, 57 (2023). https://doi.org/10.1007/s40820-023-01038-0
- C. Wang, Q. Zhang, B. Yan, B. You, J. Zheng et al., Facet engineering of advanced electrocatalysts toward hydrogen/oxygen evolution reactions. Nano Micro Lett. 15, 52 (2023). https://doi.org/10.1007/s40820-023-01024-6
- N. Danilovic, R. Subbaraman, K.C. Chang, S.H. Chang, Y.J. Kang et al., Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 5, 2474–2478 (2014). https://doi.org/10.1021/jz501061n
- C. Spöri, J.T.H. Kwan, A. Bonakdarpour, D.P. Wilkinson, P. Strasser, The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 56, 5994–6021 (2017). https://doi.org/10.1002/anie.201608601
- C.-C. Weng, X.-W. Lv, J.-T. Ren, T.-Y. Ma, Z.-Y. Yuan, Engineering gas–solid–liquid triple-phase interfaces for electrochemical energy conversion reactions. Electrochem. Energy Rev. 5, 19 (2022). https://doi.org/10.1007/s41918-022-00133-x
- H. Fan, K. Mao, M. Liu, O. Zhuo, J. Zhao et al., Tailoring the nano heterointerface of hematite/magnetite on hierarchical nitrogen-doped carbon nanocages for superb oxygen reduction. J. Mater. Chem. A 6, 21313–21319 (2018). https://doi.org/10.1039/c8ta06442c
- R.P. Forslund, W.G. Hardin, X. Rong, A.M. Abakumov, D. Filimonov et al., Exceptional electrocatalytic oxygen evolution via tunable charge transfer interactions in La0.5Sr1.5Ni1-xFexO4±δ Ruddlesden-Popper oxides. Nat. Commun. 9, 3150 (2018). https://doi.org/10.1038/s41467-018-05600-y
- D. Guan, G. Ryu, Z. Hu, J. Zhou, C.L. Dong et al., Utilizing ion leaching effects for achieving high oxygen-evolving performance on hybrid nanocomposite with self-optimized behaviors. Nat. Commun. 11, 3376 (2020). https://doi.org/10.1038/s41467-020-17108-5
- T. Wu, S. Sun, J. Song, S. Xi, Y. Du et al., Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019). https://doi.org/10.1038/s41929-019-0325-4
- J. Huang, Y. Li, R.-K. Huang, C.-T. He, L. Gong et al., Electrochemical exfoliation of pillared-layer metal-organic framework to boost the oxygen evolution reaction. Angew. Chem. Int. Ed. 57, 4632–4636 (2018). https://doi.org/10.1002/anie.201801029
- S. Jin, Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts? ACS Energy Lett. 2, 1937–1938 (2017). https://doi.org/10.1021/acsenergylett.7b00679
- X. Zou, Y. Wu, Y. Liu, D. Liu, W. Li et al., In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting. Chem 4, 1139–1152 (2018). https://doi.org/10.1016/j.chempr.2018.02.023
- Z. Wu, L. Huang, H. Liu, M. Li, H. Wang, Surface oxidation of transition metal sulfide and phosphide nanomaterials. Nano Res. 14, 2264–2267 (2021). https://doi.org/10.1007/s12274-020-3219-5
- Y. Chen, S. Ji, C. Chen, Q. Peng, D. Wang et al., Single-atom catalysts: synthetic strategies and electrochemical applications. Joule 2, 1242–1264 (2018). https://doi.org/10.1016/j.joule.2018.06.019
- A. Wang, J. Li, T. Zhang, Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018). https://doi.org/10.1038/s41570-018-0010-1
- S.K. Kaiser, Z. Chen, D. Faust Akl, S. Mitchell, J. Pérez-Ramírez, Single-atom catalysts across the priodic table. Chem. Rev. 120, 11703–11809 (2020). https://doi.org/10.1021/acs.chemrev.0c00576
- T. Sun, L. Xu, D. Wang, Y. Li, Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 12, 2067–2080 (2019). https://doi.org/10.1007/s12274-019-2345-4
- L. Bai, C.S. Hsu, D.T.L. Alexander, H.M. Chen, X. Hu, A cobalt-iron double-atom catalyst for the oxygen evolution reaction. J. Am. Chem. Soc. 141, 14190–14199 (2019). https://doi.org/10.1021/jacs.9b05268
- L. Bai, C.-S. Hsu, D.T.L. Alexander, H.M. Chen, X. Hu, Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis. Nat. Energy 6, 1054–1066 (2021). https://doi.org/10.1038/s41560-021-00925-3
- S. Lu, Y. Shi, W. Zhou, Z. Zhang, F. Wu et al., Dissolution of the heteroatom dopants and formation of ortho-quinone moieties in the doped carbon materials during water electrooxidation. J. Am. Chem. Soc. 144, 3250–3258 (2022). https://doi.org/10.1021/jacs.1c13374
- P. Yin, T. Yao, Y. Wu, L. Zheng, Y. Lin et al., Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016). https://doi.org/10.1002/anie.201604802
- J. Rossmeisl, Z.W. Qu, H. Zhu, G.J. Kroes, J.K. Nørskov, Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007). https://doi.org/10.1016/j.jelechem.2006.11.008
- O. Diaz-Morales, I. Ledezma-Yanez, M.T.M. Koper, F. Calle-Vallejo, Guidelines for the rational design of Ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal. 5, 5380–5387 (2015). https://doi.org/10.1021/acscatal.5b01638
- X.-C. Huang, Y.-Y. Lin, J.-P. Zhang, X.-M. Chen, Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 45, 1557–1559 (2006). https://doi.org/10.1002/anie.200503778
- K.S. Park, Z. Ni, A.P. Côté, J.Y. Choi, R. Huang et al., Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 103, 10186–10191 (2006). https://doi.org/10.1073/pnas.0602439103
- L. Jiao, J. Zhu, Y. Zhang, W. Yang, S. Zhou et al., Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction. J. Am. Chem. Soc. 143, 19417–19424 (2021). https://doi.org/10.1021/jacs.1c08050
- D. Liu, S. Ding, C. Wu, W. Gan, C. Wang et al., Synergistic effect of an atomically dual-metal doped catalyst for highly efficient oxygen evolution. J. Mater. Chem. A 6, 6840–6846 (2018). https://doi.org/10.1039/c8ta00550h
- L. Zhang, J. Fischer, Y. Jia, X. Yan, W. Xu et al., Coordination of atomic Co-Pt coupling species at carbon defects as active sites for oxygen reduction reaction. J. Am. Chem. Soc. 140, 10757–10763 (2018). https://doi.org/10.1021/jacs.8b04647
- Z. Lu, B. Wang, Y. Hu, W. Liu, Y. Zhao et al., An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem. Int. Ed. 58, 2622–2626 (2019). https://doi.org/10.1002/anie.201810175
- X. Li, X. Huang, S. Xi, S. Miao, J. Ding et al., Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018). https://doi.org/10.1021/jacs.8b05992
- Y. Sun, L. Silvioli, N.R. Sahraie, W. Ju, J. Li et al., Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts. J. Am. Chem. Soc. 141, 12372–12381 (2019). https://doi.org/10.1021/jacs.9b05576
- K. Jiang, S. Siahrostami, T. Zheng, Y. Hu, S. Hwang et al., Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018). https://doi.org/10.1039/c7ee03245e
- H. Fei, J. Dong, Y. Feng, C.S. Allen, C. Wan et al., General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electr ocatalytic activities. Nat. Catal. 1, 63–72 (2018). https://doi.org/10.1038/s41929-017-0008-y
- C. Zhao, X. Dai, T. Yao, W. Chen, X. Wang et al., Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017). https://doi.org/10.1021/jacs.7b02736
- Z. Zhang, X. Zhao, S. Xi, L. Zhang, Z. Chen et al., Atomically dispersed cobalt trifunctional electrocatalysts with tailored coordination environment for flexible rechargeable Zn-air battery and self-driven water splitting. Adv. Energy Mater. 10, 2002896 (2020). https://doi.org/10.1002/aenm.202002896
- X. Wang, P. Li, Z. Li, W. Chen, H. Zhou et al., 2D MOF induced accessible and exclusive Co single sites for an efficient O-silylation of alcohols with silanes. Chem. Commun. 55, 6563–6566 (2019). https://doi.org/10.1039/c9cc01717h
- T. Zhang, X. Han, H. Liu, M. Biset-Peiró, X. Zhang et al., Quasi-double-star nickel and iron active sites for high-efficiency carbon dioxide electroreduction. Energy Environ. Sci. 14, 4847–4857 (2021). https://doi.org/10.1039/d1ee01592c
- J. Yang, Z. Qiu, C. Zhao, W. Wei, W. Chen et al., In situ thermal atomization to convert supported nickel nanops into surface-bound nickel single-atom catalysts. Angew. Chem. Int. Ed. 57, 14095–14100 (2018). https://doi.org/10.1002/anie.201808049
- X. Han, X. Ling, D. Yu, D. Xie, L. Li et al., Atomically dispersed binary Co–Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 31, 1905622 (2019). https://doi.org/10.1002/adma.201905622
- Z. Pei, X.F. Lu, H. Zhang, Y. Li, D. Luan et al., Highly efficient electrocatalytic oxygen evolution over atomically dispersed synergistic Ni/Co dual sites. Angew. Chem. Int. Ed. 61, 202207527 (2022). https://doi.org/10.1002/anie.202207537
- E. Jung, H. Shin, B.H. Lee, V. Efremov, S. Lee et al., Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020). https://doi.org/10.1038/s41563-019-0571-5
- J. Song, C. Wei, Z.F. Huang, C. Liu, L. Zeng et al., A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020). https://doi.org/10.1039/c9cs00607a
- W. Wan, Y. Zhao, S. Wei, C.A. Triana, J. Li et al., Mechanistic insight into the active centers of single/dual-atom Ni/Fe-based oxygen electrocatalysts. Nat. Commun. 12, 5589 (2021). https://doi.org/10.1038/s41467-021-25811-0
- S. Zhao, C. Tan, C.-T. He, P. An, F. Xie et al., Structural transformation of highly active metal-organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 5, 881–890 (2020). https://doi.org/10.1038/s41560-020-00709-1
- H. Xu, D. Cheng, D. Cao, X.C. Zeng, A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348 (2018). https://doi.org/10.1038/s41929-018-0063-z
- J.-S. Li, Y. Wang, C.-H. Liu, S.-L. Li, Y.-G. Wang et al., Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 7, 11204 (2016). https://doi.org/10.1038/ncomms11204
- Y. Wu, W. Wei, R. Yu, L. Xia, X. Hong et al., Anchoring sub-nanometer Pt clusters on crumpled paper-like MXene enables high hydrogen evolution mass activity. Adv. Funct. Mater. 32, 2110910 (2022). https://doi.org/10.1002/adfm.202110910
- J. Guan, X. Bai, T. Tang, Recent progress and prospect of carbon-free single-site catalysts for the hydrogen and oxygen evolution reactions. Nano Res. 15, 818–837 (2021). https://doi.org/10.1007/s12274-021-3680-9
References
Y. Jiao, Y. Zheng, M. Jaroniec, S.Z. Qiao, Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015). https://doi.org/10.1039/c4cs00470a
J. Wang, Y. Gao, H. Kong, J. Kim, S. Choi et al., Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 49, 9154–9196 (2020). https://doi.org/10.1039/d0cs00575d
H.-Y. Wang, M.-L. Sun, J.-T. Ren, Z.-Y. Yuan, Circumventing challenges: design of anodic electrocatalysts for hybrid water electrolysis systems. Adv. Energy Mater. 13, 2203568 (2021). https://doi.org/10.1002/aenm.202203568
H. Fan, Y. Wang, F. Gao, L. Yang, M. Liu et al., Hierarchical sulfur and nitrogen co-doped carbon nanocages as efficient bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. J. Energy Chem. 34, 64–71 (2019). https://doi.org/10.1016/j.jechem.2018.09.003
X. Tian, P. Zhao, W. Sheng, Hydrogen evolution and oxidation: mechanistic studies and material advances. Adv. Mater. 31, 1808066 (2019). https://doi.org/10.1002/adma.201808066
S. Park, Y. Shao, J. Liu, Y. Wang, Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: status and perspective. Energy Environ. Sci. 5, 9331–9344 (2012). https://doi.org/10.1039/c2ee22554a
M. Yu, E. Budiyanto, H. Tüysüz, Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 61, 202103824 (2022). https://doi.org/10.1002/anie.202103824
J.-X. Wu, C.-T. He, G.-R. Li, J.-P. Zhang, An inorganic-MOF-inorganic approach to ultrathin CuO decorated Cu-C hybrid nanorod arrays for an efficient oxygen evolution reaction. J. Mater. Chem. A 6, 19176–19181 (2018). https://doi.org/10.1039/c8ta06069j
J.-T. Ren, Y. Yao, Z.-Y. Yuan, Fabrication strategies of porous precious-metal-free bifunctional electrocatalysts for overall water splitting: recent advances. Green Energy Environ. 6, 620–643 (2021). https://doi.org/10.1016/j.gee.2020.11.023
J.-X. Wu, P.P. Bag, Y.-T. Xu, L. Gong, C.-T. He et al., Graphene-like hydrogen-bonded melamine-cyanuric acid supramolecular nanosheets as pseudo-porous catalyst support. Adv. Mater. 33, 2007368 (2021). https://doi.org/10.1002/adma.202007368
Y. Zhang, M. Shi, C. Wang, Y. Zhu, N. Li et al., Vertically aligned NiS2/CoS2/MoS2 nanosheet array as an efficient and low-cost electrocatalyst for hydrogen evolution reaction in alkaline media. Sci. Bull. 65, 359–366 (2020). https://doi.org/10.1016/j.scib.2019.12.003
Y. Yin, Y. Zhang, T. Gao, T. Yao, X. Zhang et al., Synergistic phase and disorder engineering in 1T-MoSe2 nanosheets for enhanced hydrogen-evolution reaction. Adv. Mater. 29, 1700311 (2017). https://doi.org/10.1002/adma.201700311
W.-F. Chen, K. Sasaki, C. Ma, A.I. Frenkel, N. Marinkovic et al., Hydrogen-evolution catalysts based on non-noble metal nickel-molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012). https://doi.org/10.1002/anie.201200699
F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu et al., Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140, 7748–7759 (2018). https://doi.org/10.1021/jacs.8b04546
F. Dionigi, Z. Zeng, I. Sinev, T. Merzdorf, S. Deshpande et al., In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 11, 2522 (2020). https://doi.org/10.1038/s41467-020-16237-1
J.Y. Chen, L. Dang, H. Liang, W. Bi, J.B. Gerken et al., Operando analysis of NiFe and Fe oxyhydroxide electrocatalysts for water oxidation: detection of Fe4+ by Mössbauer spectroscopy. J. Am. Chem. Soc. 137, 15090–15093 (2015). https://doi.org/10.1021/jacs.5b10699
Y. Jia, L. Zhang, G. Gao, H. Chen, B. Wang et al., A heterostructure coupling of exfoliated Ni–Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 29, 1700017 (2017). https://doi.org/10.1002/adma.201700017
H. Wang, Z.N. Chen, D. Wu, M. Cao, F. Sun et al., Significantly enhanced overall water splitting performance by partial oxidation of Ir through Au modification in core-shell alloy structure. J. Am. Chem. Soc. 143, 4639–4645 (2021). https://doi.org/10.1021/jacs.0c12740
L. Sun, Q. Luo, Z. Dai, F. Ma, Material libraries for electrocatalytic overall water splitting. Coordin. Chem. Rev. 444, 214049 (2021). https://doi.org/10.1016/j.ccr.2021.214049
L. Chen, J.-T. Ren, Z.-Y. Yuan, Design strategies of phosphorus-containing catalysts for photocatalytic, photoelectrochemical and electrocatalytic water splittings. Green Chem. 24, 713–747 (2022). https://doi.org/10.1039/d1gc03768d
B. Guo, Y. Ding, H. Huo, X. Wen, X. Ren et al., Recent advances of transition metal basic salts for electrocatalytic oxygen evolution reaction and overall water electrolysis. Nano Micro Lett. 15, 57 (2023). https://doi.org/10.1007/s40820-023-01038-0
C. Wang, Q. Zhang, B. Yan, B. You, J. Zheng et al., Facet engineering of advanced electrocatalysts toward hydrogen/oxygen evolution reactions. Nano Micro Lett. 15, 52 (2023). https://doi.org/10.1007/s40820-023-01024-6
N. Danilovic, R. Subbaraman, K.C. Chang, S.H. Chang, Y.J. Kang et al., Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 5, 2474–2478 (2014). https://doi.org/10.1021/jz501061n
C. Spöri, J.T.H. Kwan, A. Bonakdarpour, D.P. Wilkinson, P. Strasser, The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 56, 5994–6021 (2017). https://doi.org/10.1002/anie.201608601
C.-C. Weng, X.-W. Lv, J.-T. Ren, T.-Y. Ma, Z.-Y. Yuan, Engineering gas–solid–liquid triple-phase interfaces for electrochemical energy conversion reactions. Electrochem. Energy Rev. 5, 19 (2022). https://doi.org/10.1007/s41918-022-00133-x
H. Fan, K. Mao, M. Liu, O. Zhuo, J. Zhao et al., Tailoring the nano heterointerface of hematite/magnetite on hierarchical nitrogen-doped carbon nanocages for superb oxygen reduction. J. Mater. Chem. A 6, 21313–21319 (2018). https://doi.org/10.1039/c8ta06442c
R.P. Forslund, W.G. Hardin, X. Rong, A.M. Abakumov, D. Filimonov et al., Exceptional electrocatalytic oxygen evolution via tunable charge transfer interactions in La0.5Sr1.5Ni1-xFexO4±δ Ruddlesden-Popper oxides. Nat. Commun. 9, 3150 (2018). https://doi.org/10.1038/s41467-018-05600-y
D. Guan, G. Ryu, Z. Hu, J. Zhou, C.L. Dong et al., Utilizing ion leaching effects for achieving high oxygen-evolving performance on hybrid nanocomposite with self-optimized behaviors. Nat. Commun. 11, 3376 (2020). https://doi.org/10.1038/s41467-020-17108-5
T. Wu, S. Sun, J. Song, S. Xi, Y. Du et al., Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019). https://doi.org/10.1038/s41929-019-0325-4
J. Huang, Y. Li, R.-K. Huang, C.-T. He, L. Gong et al., Electrochemical exfoliation of pillared-layer metal-organic framework to boost the oxygen evolution reaction. Angew. Chem. Int. Ed. 57, 4632–4636 (2018). https://doi.org/10.1002/anie.201801029
S. Jin, Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts? ACS Energy Lett. 2, 1937–1938 (2017). https://doi.org/10.1021/acsenergylett.7b00679
X. Zou, Y. Wu, Y. Liu, D. Liu, W. Li et al., In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting. Chem 4, 1139–1152 (2018). https://doi.org/10.1016/j.chempr.2018.02.023
Z. Wu, L. Huang, H. Liu, M. Li, H. Wang, Surface oxidation of transition metal sulfide and phosphide nanomaterials. Nano Res. 14, 2264–2267 (2021). https://doi.org/10.1007/s12274-020-3219-5
Y. Chen, S. Ji, C. Chen, Q. Peng, D. Wang et al., Single-atom catalysts: synthetic strategies and electrochemical applications. Joule 2, 1242–1264 (2018). https://doi.org/10.1016/j.joule.2018.06.019
A. Wang, J. Li, T. Zhang, Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018). https://doi.org/10.1038/s41570-018-0010-1
S.K. Kaiser, Z. Chen, D. Faust Akl, S. Mitchell, J. Pérez-Ramírez, Single-atom catalysts across the priodic table. Chem. Rev. 120, 11703–11809 (2020). https://doi.org/10.1021/acs.chemrev.0c00576
T. Sun, L. Xu, D. Wang, Y. Li, Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 12, 2067–2080 (2019). https://doi.org/10.1007/s12274-019-2345-4
L. Bai, C.S. Hsu, D.T.L. Alexander, H.M. Chen, X. Hu, A cobalt-iron double-atom catalyst for the oxygen evolution reaction. J. Am. Chem. Soc. 141, 14190–14199 (2019). https://doi.org/10.1021/jacs.9b05268
L. Bai, C.-S. Hsu, D.T.L. Alexander, H.M. Chen, X. Hu, Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis. Nat. Energy 6, 1054–1066 (2021). https://doi.org/10.1038/s41560-021-00925-3
S. Lu, Y. Shi, W. Zhou, Z. Zhang, F. Wu et al., Dissolution of the heteroatom dopants and formation of ortho-quinone moieties in the doped carbon materials during water electrooxidation. J. Am. Chem. Soc. 144, 3250–3258 (2022). https://doi.org/10.1021/jacs.1c13374
P. Yin, T. Yao, Y. Wu, L. Zheng, Y. Lin et al., Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016). https://doi.org/10.1002/anie.201604802
J. Rossmeisl, Z.W. Qu, H. Zhu, G.J. Kroes, J.K. Nørskov, Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007). https://doi.org/10.1016/j.jelechem.2006.11.008
O. Diaz-Morales, I. Ledezma-Yanez, M.T.M. Koper, F. Calle-Vallejo, Guidelines for the rational design of Ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal. 5, 5380–5387 (2015). https://doi.org/10.1021/acscatal.5b01638
X.-C. Huang, Y.-Y. Lin, J.-P. Zhang, X.-M. Chen, Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 45, 1557–1559 (2006). https://doi.org/10.1002/anie.200503778
K.S. Park, Z. Ni, A.P. Côté, J.Y. Choi, R. Huang et al., Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 103, 10186–10191 (2006). https://doi.org/10.1073/pnas.0602439103
L. Jiao, J. Zhu, Y. Zhang, W. Yang, S. Zhou et al., Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction. J. Am. Chem. Soc. 143, 19417–19424 (2021). https://doi.org/10.1021/jacs.1c08050
D. Liu, S. Ding, C. Wu, W. Gan, C. Wang et al., Synergistic effect of an atomically dual-metal doped catalyst for highly efficient oxygen evolution. J. Mater. Chem. A 6, 6840–6846 (2018). https://doi.org/10.1039/c8ta00550h
L. Zhang, J. Fischer, Y. Jia, X. Yan, W. Xu et al., Coordination of atomic Co-Pt coupling species at carbon defects as active sites for oxygen reduction reaction. J. Am. Chem. Soc. 140, 10757–10763 (2018). https://doi.org/10.1021/jacs.8b04647
Z. Lu, B. Wang, Y. Hu, W. Liu, Y. Zhao et al., An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem. Int. Ed. 58, 2622–2626 (2019). https://doi.org/10.1002/anie.201810175
X. Li, X. Huang, S. Xi, S. Miao, J. Ding et al., Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018). https://doi.org/10.1021/jacs.8b05992
Y. Sun, L. Silvioli, N.R. Sahraie, W. Ju, J. Li et al., Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts. J. Am. Chem. Soc. 141, 12372–12381 (2019). https://doi.org/10.1021/jacs.9b05576
K. Jiang, S. Siahrostami, T. Zheng, Y. Hu, S. Hwang et al., Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018). https://doi.org/10.1039/c7ee03245e
H. Fei, J. Dong, Y. Feng, C.S. Allen, C. Wan et al., General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electr ocatalytic activities. Nat. Catal. 1, 63–72 (2018). https://doi.org/10.1038/s41929-017-0008-y
C. Zhao, X. Dai, T. Yao, W. Chen, X. Wang et al., Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017). https://doi.org/10.1021/jacs.7b02736
Z. Zhang, X. Zhao, S. Xi, L. Zhang, Z. Chen et al., Atomically dispersed cobalt trifunctional electrocatalysts with tailored coordination environment for flexible rechargeable Zn-air battery and self-driven water splitting. Adv. Energy Mater. 10, 2002896 (2020). https://doi.org/10.1002/aenm.202002896
X. Wang, P. Li, Z. Li, W. Chen, H. Zhou et al., 2D MOF induced accessible and exclusive Co single sites for an efficient O-silylation of alcohols with silanes. Chem. Commun. 55, 6563–6566 (2019). https://doi.org/10.1039/c9cc01717h
T. Zhang, X. Han, H. Liu, M. Biset-Peiró, X. Zhang et al., Quasi-double-star nickel and iron active sites for high-efficiency carbon dioxide electroreduction. Energy Environ. Sci. 14, 4847–4857 (2021). https://doi.org/10.1039/d1ee01592c
J. Yang, Z. Qiu, C. Zhao, W. Wei, W. Chen et al., In situ thermal atomization to convert supported nickel nanops into surface-bound nickel single-atom catalysts. Angew. Chem. Int. Ed. 57, 14095–14100 (2018). https://doi.org/10.1002/anie.201808049
X. Han, X. Ling, D. Yu, D. Xie, L. Li et al., Atomically dispersed binary Co–Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 31, 1905622 (2019). https://doi.org/10.1002/adma.201905622
Z. Pei, X.F. Lu, H. Zhang, Y. Li, D. Luan et al., Highly efficient electrocatalytic oxygen evolution over atomically dispersed synergistic Ni/Co dual sites. Angew. Chem. Int. Ed. 61, 202207527 (2022). https://doi.org/10.1002/anie.202207537
E. Jung, H. Shin, B.H. Lee, V. Efremov, S. Lee et al., Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020). https://doi.org/10.1038/s41563-019-0571-5
J. Song, C. Wei, Z.F. Huang, C. Liu, L. Zeng et al., A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020). https://doi.org/10.1039/c9cs00607a
W. Wan, Y. Zhao, S. Wei, C.A. Triana, J. Li et al., Mechanistic insight into the active centers of single/dual-atom Ni/Fe-based oxygen electrocatalysts. Nat. Commun. 12, 5589 (2021). https://doi.org/10.1038/s41467-021-25811-0
S. Zhao, C. Tan, C.-T. He, P. An, F. Xie et al., Structural transformation of highly active metal-organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 5, 881–890 (2020). https://doi.org/10.1038/s41560-020-00709-1
H. Xu, D. Cheng, D. Cao, X.C. Zeng, A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348 (2018). https://doi.org/10.1038/s41929-018-0063-z
J.-S. Li, Y. Wang, C.-H. Liu, S.-L. Li, Y.-G. Wang et al., Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 7, 11204 (2016). https://doi.org/10.1038/ncomms11204
Y. Wu, W. Wei, R. Yu, L. Xia, X. Hong et al., Anchoring sub-nanometer Pt clusters on crumpled paper-like MXene enables high hydrogen evolution mass activity. Adv. Funct. Mater. 32, 2110910 (2022). https://doi.org/10.1002/adfm.202110910
J. Guan, X. Bai, T. Tang, Recent progress and prospect of carbon-free single-site catalysts for the hydrogen and oxygen evolution reactions. Nano Res. 15, 818–837 (2021). https://doi.org/10.1007/s12274-021-3680-9