Shape-Controlled Synthesis of Platinum-Based Nanocrystals and Their Electrocatalytic Applications in Fuel Cells
Corresponding Author: Jiye Fang
Nano-Micro Letters,
Vol. 15 (2023), Article Number: 83
Abstract
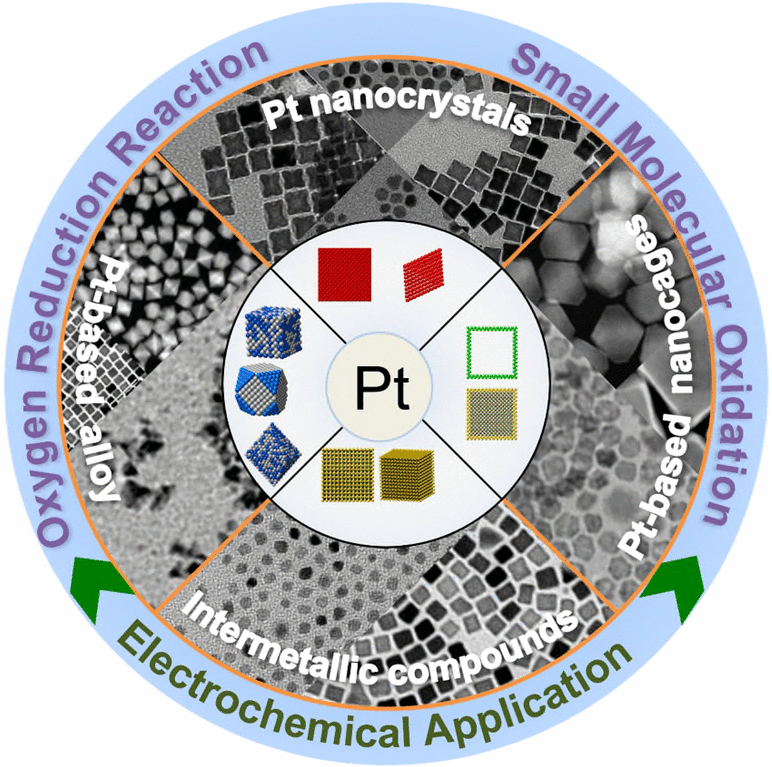
To achieve environmentally benign energy conversion with the carbon neutrality target via electrochemical reactions, the innovation of electrocatalysts plays a vital role in the enablement of renewable resources. Nowadays, Pt-based nanocrystals (NCs) have been identified as one class of the most promising candidates to efficiently catalyze both the half-reactions in hydrogen- and hydrocarbon-based fuel cells. Here, we thoroughly discuss the key achievement in developing shape-controlled Pt and Pt-based NCs, and their electrochemical applications in fuel cells. We begin with a mechanistic discussion on how the morphology can be precisely controlled in a colloidal system, followed by highlighting the advanced development of shape-controlled Pt, Pt-alloy, Pt-based core@shell NCs, Pt-based nanocages, and Pt-based intermetallic compounds. We then select some case studies on models of typical reactions (oxygen reduction reaction at the cathode and small molecular oxidation reaction at the anode) that are enhanced by the shape-controlled Pt-based nanocatalysts. Finally, we provide an outlook on the potential challenges of shape-controlled nanocatalysts and envision their perspective with suggestions.
Highlights:
1 Synthetic mechanisms of shape-controlled Pt-based alloy and intermetallic nanocrystals are outlined, and strategies for the design and development of morphology-controlled Pt-based nanostructures are discussed.
2 Advanced characterizations and electrochemical applications of these Pt-based nanocatalysts are highlighted.
3 Advances and perspectives in designing outperformance and the long-durability of Pt-based nanocatalysts with shape control in this electrochemical field are proposed.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- K.D. Gilroy, A. Ruditskiy, H.-C. Peng, D. Qin, Y. Xia, Bimetallic nanocrystals: syntheses, properties, and applications. Chem. Rev. 116(18), 10414–10472 (2016). https://doi.org/10.1021/acs.chemrev.6b00211
- M. Zhou, C. Li, J. Fang, Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications. Chem. Rev. 121(2), 736–795 (2021). https://doi.org/10.1021/acs.chemrev.0c00436
- C. Li, S. Yan, J. Fang, Construction of lattice strain in bimetallic nanostructures and its effectiveness in electrochemical applications. Small 17(46), 2102244 (2021). https://doi.org/10.1002/smll.202102244
- Y. Xia, Y. Xiong, B. Lim, S.E. Skrabalak, Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48(1), 60–103 (2009). https://doi.org/10.1002/anie.200802248
- N.S. Porter, H. Wu, Z. Quan, J. Fang, Shape-control and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc. Chem. Res. 46(8), 1867–1877 (2013). https://doi.org/10.1021/ar3002238
- A.R. Tao, S. Habas, P. Yang, Shape control of colloidal metal nanocrystals. Small 4(3), 310–325 (2008). https://doi.org/10.1002/smll.200701295
- Y. Kang, C.B. Murray, Synthesis and electrocatalytic properties of cubic Mn−Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 132(22), 7568–7569 (2010). https://doi.org/10.1021/ja100705j
- Y. Kang, J.B. Pyo, X. Ye, T.R. Gordon, C.B. Murray, Synthesis, shape control, and methanol electro-oxidation properties of Pt-Zn alloy and Pt3Zn intermetallic nanocrystals. ACS Nano 6(6), 5642–5647 (2012). https://doi.org/10.1021/nn301583g
- B. Arumugam, T. Tamaki, T. Yamaguchi, Beneficial role of copper in the enhancement of durability of ordered intermetallic PtFeCu catalyst for electrocatalytic oxygen reduction. ACS Appl. Mater. Interfaces 7(30), 16311–16321 (2015). https://doi.org/10.1021/acsami.5b03137
- D. Wang, H.L. Xin, R. Hovden, H. Wang, Y. Yu et al., Structurally ordered intermetallic platinum-cobalt core-shell nanops with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12(1), 81–87 (2013). https://doi.org/10.1038/nmat3458
- J. Li, S. Sun, Intermetallic nanops: synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 52(7), 2015–2025 (2019). https://doi.org/10.1021/acs.accounts.9b00172
- H.S. Chen, T.M. Benedetti, V.R. Goncales, N.M. Bedford, R.W.J. Scott et al., Preserving the exposed facets of Pt3Sn intermetallic nanocubes during an order to disorder transition allows the elucidation of the effect of the degree of alloy ordering on electrocatalysis. J. Am. Chem. Soc. 142(6), 3231–3239 (2020). https://doi.org/10.1021/jacs.9b13313
- V.R. Stamenkovic, B. Fowler, B.S. Mun, G. Wang, P.N. Ross et al., Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315(5811), 493–497 (2007). https://doi.org/10.1126/science.1135941
- V.R. Stamenkovic, B.S. Mun, M. Arenz, K.J.J. Mayrhofer, C.A. Lucas et al., Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6(3), 241–247 (2007). https://doi.org/10.1038/nmat1840
- J. Greeley, I.E.L. Stephens, A.S. Bondarenko, T.P. Johansson, H.A. Hansen et al., Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1(7), 552–556 (2009). https://doi.org/10.1038/nchem.367
- J. Wu, A. Gross, H. Yang, Shape and composition-controlled platinum alloy nanocrystals using carbon monoxide as reducing agent. Nano Lett. 11(2), 798–802 (2011). https://doi.org/10.1021/nl104094p
- J. Zhang, H. Yang, J. Fang, S. Zou, Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 10(2), 638–644 (2010). https://doi.org/10.1021/nl903717z
- Z. Ma, Z.P. Cano, A. Yu, Z. Chen, G. Jiang et al., Enhancing oxygen reduction activity of Pt-based electrocatalysts: from theoretical mechanisms to practical methods. Angew. Chem. Int. Ed. 59(42), 18334–18348 (2020). https://doi.org/10.1002/anie.202003654
- F. Kong, Z. Ren, M.N. Banis, L. Du, X. Zhou et al., Active and stable Pt–Ni alloy octahedra catalyst for oxygen reduction via near-surface atomical engineering. ACS Catal. 10(7), 4205–4214 (2020). https://doi.org/10.1021/acscatal.9b05133
- M. Nunez, J.L. Lansford, D.G. Vlachos, Optimization of the facet structure of transition-metal catalysts applied to the oxygen reduction reaction. Nat. Chem. 11(5), 449–456 (2019). https://doi.org/10.1038/s41557-019-0247-4
- X. Huang, Z. Zhao, L. Cao, Y. Chen, E. Zhu et al., High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348(6240), 1230–1234 (2015). https://doi.org/10.1126/science.aaa8765
- S.I. Choi, S. Xie, M. Shao, J.H. Odell, N. Lu et al., Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mgPt for the oxygen reduction reaction. Nano Lett. 13(7), 3420–3425 (2013). https://doi.org/10.1021/nl401881z
- H. Frey, A. Beck, X. Huang, J.A. van Bokhoven, M.G. Willinger, Dynamic interplay between metal nanops and oxide support under redox conditions. Science 376(6596), 982–987 (2022). https://doi.org/10.1126/science.abm3371
- Z. Luo, G. Zhao, H. Pan, W. Sun, Strong metal–support interaction in heterogeneous catalysts. Adv. Energy Mater. 12(37), 2201395 (2022). https://doi.org/10.1002/aenm.202201395
- A. Shan, X. Teng, Y. Zhang, P. Zhang, Y. Xu et al., Interfacial electronic structure modulation of Pt-MoS2 heterostructure for enhancing electrocatalytic hydrogen evolution reaction. Nano Energy 94, 106913 (2022). https://doi.org/10.1016/j.nanoen.2021.106913
- H. Mistry, A.S. Varela, S. Kühl, P. Strasser, B.R. Cuenya, Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1(4), 16009 (2016). https://doi.org/10.1038/natrevmats.2016.9
- P. Yin, S.-C. Shen, L.-L. Zhang, X.-S. Zheng, M. Zuo et al., Ultra-high-temperature strong metal-support interactions in carbon-supported catalysts. Cell Rep. Phys. Sci. 3(8), 100984 (2022). https://doi.org/10.1016/j.xcrp.2022.100984
- Y.T. Guntern, V. Okatenko, J. Pankhurst, S.B. Varandili, P. Iyengar et al., Colloidal nanocrystals as electrocatalysts with tunable activity and selectivity. ACS Catal. 11(3), 1248–1295 (2021). https://doi.org/10.1021/acscatal.0c04403
- Y. Xia, X. Xia, H.-C. Peng, Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 137(25), 7947–7966 (2015). https://doi.org/10.1021/jacs.5b04641
- C. Wang, C. Lin, L. Zhang, Z. Quan, K. Sun et al., Pt3Co concave nanocubes: synthesis, formation understanding, and enhanced catalytic activity toward hydrogenation of styrene. Chem. Eur. J. 20(6), 1753–1759 (2014). https://doi.org/10.1002/chem.201301724
- J. Zhang, J. Fang, A general strategy for preparation of Pt 3d-transition metal (Co, Fe, Ni) nanocubes. J. Am. Chem. Soc. 131(51), 18543–18547 (2009). https://doi.org/10.1021/ja908245r
- C. Wang, C. Lin, B. Zhao, L. Zhang, A. Kumbhar et al., High-indexed Pt3Fe nanocatalysts and their enhanced catalytic performance in dual organic reactions. ChemNanoMat. 1(5), 331–337 (2015). https://doi.org/10.1002/cnma.201500048
- C. Li, J. Pan, L. Zhang, J. Fang, Colloidal synthesis of monodisperse trimetallic Pt–Fe–Ni nanocrystals and their enhanced electrochemical performances. Nanotechnology 34(7), 075401 (2023). https://doi.org/10.1088/1361-6528/aca337
- Y. Kang, X. Ye, C.B. Murray, Size- and shape-selective synthesis of metal nanocrystals and nanowires using co as a reducing agent. Angew. Chem. Int. Ed. 49(35), 6156–6159 (2010). https://doi.org/10.1002/anie.201003383
- M. Chen, J. Kim, J.P. Liu, H. Fan, S. Sun, Synthesis of FePt nanocubes and their oriented self-assembly. J. Am. Chem. Soc. 128(22), 7132–7133 (2006). https://doi.org/10.1021/ja061704x
- S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Monodisperse FePt nanops and ferromagnetic FePt nanocrystal superlattices. Science 287(5460), 1989–1992 (2000). https://doi.org/10.1126/science.287.5460.1989
- D. Xu, Z. Liu, H. Yang, Q. Liu, J. Zhang et al., Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum–copper nanocubes. Angew. Chem. Int. Ed. 48(23), 4217–4221 (2009). https://doi.org/10.1002/anie.200900293
- G. Almeida, L. Goldoni, Q. Akkerman, Z. Dang, A.H. Khan et al., Role of acid-base equilibria in the size, shape, and phase control of cesium lead bromide nanocrystals. ACS Nano 12(2), 1704–1711 (2018). https://doi.org/10.1021/acsnano.7b08357
- C. Chen, Y. Kang, Z. Huo, Z. Zhu, W. Huang et al., Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343(6177), 1339–1343 (2014). https://doi.org/10.1126/science.1249061
- J. Zhang, H. Yang, B. Martens, Z. Luo, D. Xu et al., Pt–Cu nanoctahedra: synthesis and comparative study with nanocubes on their electrochemical catalytic performance. Chem. Sci. 3(11), 3302–3306 (2012). https://doi.org/10.1039/C2SC20514A
- Y. Wang, J. He, C. Liu, W.H. Chong, H. Chen, Thermodynamics versus kinetics in nanosynthesis. Angew. Chem. Int. Ed. 54(7), 2022–2051 (2015). https://doi.org/10.1002/anie.201402986
- N. Pradhan, D. Reifsnyder, R. Xie, J. Aldana, X. Peng, Surface ligand dynamics in growth of nanocrystals. J. Am. Chem. Soc. 129(30), 9500–9509 (2007). https://doi.org/10.1021/ja0725089
- R. Costi, A.E. Saunders, U. Banin, Colloidal hybrid nanostructures: a new type of functional materials. Angew. Chem. Int. Ed. 49(29), 4878–4897 (2010). https://doi.org/10.1002/anie.200906010
- Y. Wang, H.-C. Peng, J. Liu, C.Z. Huang, Y. Xia, Use of reduction rate as a quantitative knob for controlling the twin structure and shape of palladium nanocrystals. Nano Lett. 15(2), 1445–1450 (2015). https://doi.org/10.1021/acs.nanolett.5b00158
- V.K. LaMer, R.H. Dinegar, Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 72(11), 4847–4854 (1950). https://doi.org/10.1021/ja01167a001
- S. Xie, S.-I. Choi, N. Lu, L.T. Roling, J.A. Herron et al., Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 14(6), 3570–3576 (2014). https://doi.org/10.1021/nl501205j
- G. Wulff, On the question of the rate of growth and dissolution of crystal surfaces. Z. Kristallogr. Mineral. 34, 449–530 (1901)
- P.J.M. Smeets, K.R. Cho, R.G.E. Kempen, N.A.J.M. Sommerdijk, J.J. De Yoreo, Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat. Mater. 14(4), 394–399 (2015). https://doi.org/10.1038/nmat4193
- H.G. Liao, D. Zherebetskyy, H. Xin, C. Czarnik, P. Ercius et al., Facet development during platinum nanocube growth. Science 345(6199), 916–919 (2014). https://doi.org/10.1126/science.1253149
- D. Li, M.H. Nielsen, J.R.I. Lee, C. Frandsen, J.F. Banfield et al., Direction-specific interactions control crystal growth by oriented attachment. Science 336(6084), 1014–1018 (2012). https://doi.org/10.1126/science.1219643
- H. Zheng, R.K. Smith, Y.-W. Jun, C. Kisielowski, U. Dahmen et al., Observation of single colloidal platinum nanocrystal growth trajectories. Science 324(5932), 1309–1312 (2009). https://doi.org/10.1126/science.1172104
- P.L. Hansen, J.B. Wagner, S. Helveg, J.R. Rostrup-Nielsen, B.S. Clausen et al., Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295(5562), 2053–2055 (2002). https://doi.org/10.1126/science.1069325
- H.-G. Liao, L. Cui, S. Whitelam, H. Zheng, Real-time imaging of Pt3Fe nanorod growth in solution. Science 336(6084), 1011–1014 (2012). https://doi.org/10.1126/science.1219185
- Z. Zeng, W. Zheng, H. Zheng, Visualization of colloidal nanocrystal formation and electrode–electrolyte interfaces in liquids using TEM. Acc. Chem. Res. 50(8), 1808–1817 (2017). https://doi.org/10.1021/acs.accounts.7b00161
- X. Ye, M.R. Jones, L.B. Frechette, Q. Chen, A.S. Powers et al., Single-p mapping of nonequilibrium nanocrystal transformations. Science 354(6314), 874–877 (2016). https://doi.org/10.1126/science.aah4434
- R. Long, S. Zhou, B.J. Wiley, Y. Xiong, Oxidative etching for controlled synthesis of metal nanocrystals: atomic addition and subtraction. Chem. Soc. Rev. 43(17), 6288–6310 (2014). https://doi.org/10.1039/C4CS00136B
- Y. Jiang, G. Zhu, F. Lin, H. Zhang, C. Jin et al., In situ study of oxidative etching of palladium nanocrystals by liquid cell electron microscopy. Nano Lett. 14(7), 3761–3765 (2014). https://doi.org/10.1021/nl500670q
- Q. Zheng, J. Shangguan, X. Li, Q. Zhang, K.C. Bustillo et al., Observation of surface ligands-controlled etching of palladium nanocrystals. Nano Lett. 21(15), 6640–6647 (2021). https://doi.org/10.1021/acs.nanolett.1c02104
- H.-D. Yu, M.D. Regulacio, E. Ye, M.-Y. Han, Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 42(14), 6006–6018 (2013). https://doi.org/10.1039/C3CS60113G
- M. Azharuddin, G.H. Zhu, D. Das, E. Ozgur, L. Uzun et al., A repertoire of biomedical applications of noble metal nanops. Chem. Commun. 55(49), 6964–6996 (2019). https://doi.org/10.1039/C9CC01741K
- B.C. Steele, A. Heinzel, Materials for fuel-cell technologies. Nature 414(6861), 345–352 (2001). https://doi.org/10.1038/35104620
- J. Chen, P. Mela, M. Möller, M.C. Lensen, Microcontact deprinting: a technique to pattern gold nanops. ACS Nano 3(6), 1451–1456 (2009). https://doi.org/10.1021/nn9002924
- G. Walters, I.P. Parkin, The incorporation of noble metal nanops into host matrix thin films: synthesis, characterisation and applications. J. Mater. Chem. 19(5), 574–590 (2009). https://doi.org/10.1039/B809646E
- L. Wang, D. Ma, C. Guo, X. Jiang, M. Li et al., CsPbBr 3 nanocrystals prepared by high energy ball milling in one-step and structural transformation from CsPbBr3 to CsPb2Br5. Appl. Surf. Sci. 543, 148782 (2021). https://doi.org/10.1016/j.apsusc.2020.148782
- Y. Wang, Y. Xia, Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 4(10), 2047–2050 (2004). https://doi.org/10.1021/nl048689j
- S.E. Skrabalak, J. Chen, Y. Sun, X. Lu, L. Au et al., Gold nanocages: synthesis, properties, and applications. Acc. Chem. Res. 41(12), 1587–1595 (2008). https://doi.org/10.1021/ar800018v
- C. Wang, L. Zhang, H. Yang, J. Pan, J. Liu et al., High-indexed Pt3Ni alloy tetrahexahedral nanoframes evolved through preferential CO etching. Nano Lett. 17(4), 2204–2210 (2017). https://doi.org/10.1021/acs.nanolett.6b04731
- L. Gan, M. Heggen, S. Rudi, P. Strasser, Core–shell compositional fine structures of dealloyed PtxNi1–x nanops and their impact on oxygen reduction catalysis. Nano Lett. 12(10), 5423–5430 (2012). https://doi.org/10.1021/nl302995z
- M. Oezaslan, M. Heggen, P. Strasser, Size-dependent morphology of dealloyed bimetallic catalysts: linking the nano to the macro scale. J. Am. Chem. Soc. 134(1), 514–524 (2012). https://doi.org/10.1021/ja2088162
- L. Gan, M. Heggen, R. O’Malley, B. Theobald, P. Strasser, Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 13(3), 1131–1138 (2013). https://doi.org/10.1021/nl304488q
- Y.N. Tan, J.Y. Lee, D.I.C. Wang, Uncovering the design rules for peptide synthesis of metal nanops. J. Am. Chem. Soc. 132(16), 5677–5686 (2010). https://doi.org/10.1021/ja907454f
- C.B.M.C.R. Kagan, M.G. Bawendi, Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 30(1), 545–610 (2000). https://doi.org/10.1146/annurev.matsci.30.1.545
- Y. Wu, D. Wang, Z. Niu, P. Chen, G. Zhou et al., A strategy for designing a concave Pt-Ni alloy through controllable chemical etching. Angew. Chem. Int. Ed. 51(50), 12524–12528 (2012). https://doi.org/10.1002/anie.201207491
- J. Gu, Y.-W. Zhang, F. Tao, Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 41(24), 8050–8065 (2012). https://doi.org/10.1039/C2CS35184F
- Y. Wang, Z. Sun, A. Kumbhar, Z. Luo, C. Wang et al., Is CO adequate to facilitate the formation of Pt3M (M = Fe, Ni and Co) nanocubes? Chem. Commun. 49(38), 3955–3957 (2013). https://doi.org/10.1039/C3CC41424H
- C.-K. Tsung, J.N. Kuhn, W. Huang, C. Aliaga, L.-I. Hung et al., Sub-10 nm platinum nanocrystals with size and shape control: catalytic study for ethylene and pyrrole hydrogenation. J. Am. Chem. Soc. 131(16), 5816–5822 (2009). https://doi.org/10.1021/ja809936n
- J. Qian, M. Shen, S. Zhou, C.-T. Lee, M. Zhao et al., Synthesis of Pt nanocrystals with different shapes using the same protocol to optimize their catalytic activity toward oxygen reduction. Mater. Today 21(8), 834–844 (2018). https://doi.org/10.1016/j.mattod.2018.08.005
- W. Zhu, A.X. Yin, Y.W. Zhang, C.H. Yan, Highly shape-selective synthesis of monodispersed fivefold twinned platinum nanodecahedrons and nanoicosahedrons. Chem. Eur. J. 18(39), 12222–12226 (2012). https://doi.org/10.1002/chem.201201099
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding, Z.L. Wang, Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316(5825), 732–735 (2007). https://doi.org/10.1126/science.1140484
- L. Huang, M. Liu, H. Lin, Y. Xu, J. Wu et al., Shape regulation of high-index facet nanops by dealloying. Science 365(6458), 1159–1163 (2019). https://doi.org/10.1126/science.aax5843
- Y. Kang, J.B. Pyo, X. Ye, R.E. Diaz, T.R. Gordon et al., Shape-controlled synthesis of Pt nanocrystals: the role of metal carbonyls. ACS Nano 7(1), 645–653 (2013). https://doi.org/10.1021/nn3048439
- C. Wang, H. Daimon, Y. Lee, J. Kim, S. Sun, Synthesis of monodisperse Pt nanocubes and their enhanced catalysis for oxygen reduction. J. Am. Chem. Soc. 129(22), 6974–6975 (2007). https://doi.org/10.1021/ja070440r
- J. Ren, R.D. Tilley, Shape-controlled growth of platinum nanops. Small 3(9), 1508–1512 (2007). https://doi.org/10.1002/smll.200700135
- C.-T. Lee, X. Yang, M. Vara, K.D. Gilroy, Y. Xia, Water-based synthesis of sub-10 nm Pt octahedra and their performance towards the oxygen reduction reaction. ChemNanoMat 3(12), 879–884 (2017). https://doi.org/10.1002/cnma.201700189
- W. Zhou, J. Wu, H. Yang, Highly uniform platinum icosahedra made by hot injection-assisted grails method. Nano Lett. 13(6), 2870–2874 (2013). https://doi.org/10.1021/nl401214d
- J. Zhang, Z. Luo, B. Martens, Z. Quan, A. Kumbhar et al., Reversible kirkwood–alder transition observed in Pt3Cu2 nanoctahedron assemblies under controlled solvent annealing/drying conditions. J. Am. Chem. Soc. 134(34), 14043–14049 (2012). https://doi.org/10.1021/ja304108n
- J. Ding, L. Bu, S. Guo, Z. Zhao, E. Zhu et al., Morphology and phase controlled construction of Pt–Ni nanostructures for efficient electrocatalysis. Nano Lett. 16(4), 2762–2767 (2016). https://doi.org/10.1021/acs.nanolett.6b00471
- S. Chen, M. Li, M. Gao, J. Jin, M.A. van Spronsen et al., High-performance Pt–Co nanoframes for fuel-cell electrocatalysis. Nano Lett. 20(3), 1974–1979 (2020). https://doi.org/10.1021/acs.nanolett.9b05251
- C. Xie, Z. Niu, D. Kim, M. Li, P. Yang, Surface and interface control in nanop catalysis. Chem. Rev. 120(2), 1184–1249 (2020). https://doi.org/10.1021/acs.chemrev.9b00220
- J.R. Greer, Nanoframe catalysts. Science 343(6177), 1319–1320 (2014). https://doi.org/10.1126/science.1251865
- Q. Chen, Z. Cao, G. Du, Q. Kuang, J. Huang et al., Excavated octahedral Pt-Co alloy nanocrystals built with ultrathin nanosheets as superior multifunctional electrocatalysts for energy conversion applications. Nano Energy 39, 582–589 (2017). https://doi.org/10.1016/j.nanoen.2017.07.041
- C. Cui, L. Gan, M. Heggen, S. Rudi, P. Strasser, Compositional segregation in shaped Pt alloy nanops and their structural behaviour during electrocatalysis. Nat. Mater. 12(8), 765–771 (2013). https://doi.org/10.1038/nmat3668
- S.E. Habas, H. Lee, V. Radmilovic, G.A. Somorjai, P. Yang, Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 6(9), 692–697 (2007). https://doi.org/10.1038/nmat1957
- F.-R. Fan, D.-Y. Liu, Y.-F. Wu, S. Duan, Z.-X. Xie et al., Epitaxial growth of heterogeneous metal nanocrystals: from gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 130(22), 6949–6951 (2008). https://doi.org/10.1021/ja801566d
- T. Lv, X. Yang, Y. Zheng, H. Huang, L. Zhang et al., Controlling the growth of Au on icosahedral seeds of Pd by manipulating the reduction kinetics. J. Phys. Chem. C 120(37), 20768–20774 (2016). https://doi.org/10.1021/acs.jpcc.6b02022
- M. Yang, W. Wang, K.D. Gilroy, Y. Xia, Controlling the deposition of Pd on au nanocages: outer surface only versus both outer and inner surfaces. Nano Lett. 17(9), 5682–5687 (2017). https://doi.org/10.1021/acs.nanolett.7b02578
- L. Zhang, L.T. Roling, X. Wang, M. Vara, M. Chi et al., Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349(6246), 412–416 (2015). https://doi.org/10.1126/science.aab0801
- J. Park, L. Zhang, S.-I. Choi, L.T. Roling, N. Lu et al., Atomic layer-by-layer deposition of platinum on palladium octahedra for enhanced catalysts toward the oxygen reduction reaction. ACS Nano 9(3), 2635–2647 (2015). https://doi.org/10.1021/nn506387w
- H. Zhang, M. Jin, J. Wang, W. Li, P.H.C. Camargo et al., Synthesis of Pd−Pt bimetallic nanocrystals with a concave structure through a bromide-induced galvanic replacement reaction. J. Am. Chem. Soc. 133(15), 6078–6089 (2011). https://doi.org/10.1021/ja201156s
- X. Wang, M. Vara, M. Luo, H. Huang, A. Ruditskiy et al., Pd@Pt core–shell concave decahedra: a class of catalysts for the oxygen reduction reaction with enhanced activity and durability. J. Am. Chem. Soc. 137(47), 15036–15042 (2015). https://doi.org/10.1021/jacs.5b10059
- X. Wang, S.-I. Choi, L.T. Roling, M. Luo, C. Ma et al., Palladium–platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 6, 7594 (2015). https://doi.org/10.1038/ncomms8594
- X. Wang, L. Figueroa-Cosme, X. Yang, M. Luo, J. Liu et al., Pt-based icosahedral nanocages: using a combination of 111 facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 16(2), 1467–1471 (2016). https://doi.org/10.1021/acs.nanolett.5b05140
- D.S. He, D. He, J. Wang, Y. Lin, P. Yin et al., Ultrathin icosahedral Pt-enriched nanocage with excellent oxygen reduction reaction activity. J. Am. Chem. Soc. 138(5), 1494–1497 (2016). https://doi.org/10.1021/jacs.5b12530
- B.Y. Xia, H.B. Wu, X. Wang, X.W. Lou, One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 134(34), 13934–13937 (2012). https://doi.org/10.1021/ja3051662
- X. Tian, X. Zhao, Y.-Q. Su, L. Wang, H. Wang et al., Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366(6467), 850–856 (2019). https://doi.org/10.1126/science.aaw7493
- F. Saleem, Z. Zhang, X. Cui, Y. Gong, B. Chen et al., Elemental segregation in multimetallic core–shell nanoplates. J. Am. Chem. Soc. 141(37), 14496–14500 (2019). https://doi.org/10.1021/jacs.9b05197
- S. Zhang, Y. Hao, D. Su, V.V.T. Doan-Nguyen, Y. Wu et al., Monodisperse core/shell Ni/FePt nanops and their conversion to Ni/Pt to catalyze oxygen reduction. J. Am. Chem. Soc. 136(45), 15921–15924 (2014). https://doi.org/10.1021/ja5099066
- C. Li, X. Chen, L. Zhang, S. Yan, A. Sharma et al., Synthesis of core@shell Cu-Ni@Pt-Cu nano-octahedra and their improved MOR activity. Angew. Chem. Int. Ed. 60(14), 7675–7680 (2021). https://doi.org/10.1002/anie.202014144
- G. Liu, W. Zhou, Y. Ji, B. Chen, G. Fu et al., Hydrogen-intercalation-induced lattice expansion of Pd@Pt core–shell nanops for highly efficient electrocatalytic alcohol oxidation. J. Am. Chem. Soc. 143(29), 11262–11270 (2021). https://doi.org/10.1021/jacs.1c05856
- M. Vara, X. Wang, J. Howe, M. Chi, Y. Xia, Understanding the stability of Pt-based nanocages under thermal stress using in situ electron microscopy. ChemNanoMat 4(1), 112–117 (2018). https://doi.org/10.1002/cnma.201700298
- J.T.L. Gamler, H.M. Ashberry, S.E. Skrabalak, K.M. Koczkur, Random alloyed versus intermetallic nanops: a comparison of electrocatalytic performance. Adv. Mater. (2018). https://doi.org/10.1002/adma.201801563
- J. Li, Z. Xi, Y.-T. Pan, J.S. Spendelow, P.N. Duchesne et al., Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanops for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 140(8), 2926–2932 (2018). https://doi.org/10.1021/jacs.7b12829
- Q. Li, L. Wu, G. Wu, D. Su, H. Lv et al., New approach to fully ordered fct-FePt nanops for much enhanced electrocatalysis in acid. Nano Lett. 15(4), 2468–2473 (2015). https://doi.org/10.1021/acs.nanolett.5b00320
- M. Xie, Z. Lyu, R. Chen, M. Shen, Z. Cao et al., Pt-Co@Pt octahedral nanocrystals: enhancing their activity and durability toward oxygen reduction with an intermetallic core and an ultrathin shell. J. Am. Chem. Soc. 143(22), 8509–8518 (2021). https://doi.org/10.1021/jacs.1c04160
- J. Li, S. Sharma, K. Wei, Z. Chen, D. Morris et al., Anisotropic strain tuning of L10 ternary nanops for oxygen reduction. J. Am. Chem. Soc. 142(45), 19209–19216 (2020). https://doi.org/10.1021/jacs.0c08962
- J. Li, S. Sharma, X. Liu, Y.-T. Pan, J.S. Spendelow et al., Hard-magnet L10-CoPt nanops advance fuel cell catalysis. Joule. 3(1), 124–135 (2019). https://doi.org/10.1016/j.joule.2018.09.016
- X. Chen, S. Zhang, C. Li, Z. Liu, X. Sun et al., Composition-dependent ordering transformations in Pt–Fe nanoalloys. Proc. Natl. Acad. Sci. 119(14), e2117899119 (2022). https://doi.org/10.1073/pnas.2117899119
- Y. Qin, M. Luo, Y. Sun, C. Li, B. Huang et al., Intermetallic hcp-PtBi/fcc-Pt core/shell nanoplates enable efficient bifunctional oxygen reduction and methanol oxidation electrocatalysis. ACS Catal. 8(6), 5581–5590 (2018). https://doi.org/10.1021/acscatal.7b04406
- Q. Feng, S. Zhao, D. He, S. Tian, L. Gu et al., Strain engineering to enhance the electrooxidation performance of atomic-layer pt on intermetallic Pt3Ga. J. Am. Chem. Soc. 140(8), 2773–2776 (2018). https://doi.org/10.1021/jacs.7b13612
- L. Bu, N. Zhang, S. Guo, X. Zhang, J. Li et al., Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354(6318), 1410–1414 (2016). https://doi.org/10.1126/science.aah6133
- S. Maksimuk, S. Yang, Z. Peng, H. Yang, Synthesis and characterization of ordered intermetallic PtPb nanorods. J. Am. Chem. Soc. 129(28), 8684–8685 (2007). https://doi.org/10.1021/ja071980r
- H. Rong, J. Mao, P. Xin, D. He, Y. Chen et al., Kinetically controlling surface structure to construct defect-rich intermetallic nanocrystals: effective and stable catalysts. Adv. Mater. 28(13), 2540–2546 (2016). https://doi.org/10.1002/adma.201504831
- H. Yang, Y. Tang, S. Zou, Electrochemical removal of surfactants from Pt nanocubes. Electrochem. Commun. 38, 134–137 (2014). https://doi.org/10.1016/j.elecom.2013.11.019
- P. Godbold, G. Johnson, A.D. Obi, R. Brown, S. Hwang et al., Surfactant removal for colloidal nanocrystal catalysts mediated by n-heterocyclic carbenes. J. Am. Chem. Soc. 143(7), 2644–2648 (2021). https://doi.org/10.1021/jacs.0c12278
- W.F. Fu, Y. Shi, L. Wang, M.M. Shi, H.Y. Li et al., A green, low-cost, and highly effective strategy to enhance the performance of hybrid solar cells: post-deposition ligand exchange by acetic acid. Sol. Energy Mater. Sol. Cells. 117, 329–335 (2013). https://doi.org/10.1016/j.solmat.2013.06.042
- Z. Zhang, M. Chi, G.M. Veith, P. Zhang, D.A. Lutterman et al., Rational design of Bi nanops for efficient electrochemical CO2 reduction: the elucidation of size and surface condition effects. ACS Catal. 6(9), 6255–6264 (2016). https://doi.org/10.1021/acscatal.6b01297
- M. Zhou, H. Wang, L. Zhang, C. Li, A. Kumbhar et al., Facet impact of CuMn2O4 spinel nanocatalysts on enhancement of the oxygen reduction reaction in alkaline media. ACS Catal. 12(21), 13663–13670 (2022). https://doi.org/10.1021/acscatal.2c03275
- R. Latsuzbaia, E. Negro, G. Koper, Synthesis, stabilization and activation of pt nanops for PEMFC applications. Fuel Cells 15(4), 628–638 (2015). https://doi.org/10.1002/fuce.201500023
- M. Cargnello, C. Chen, B.T. Diroll, V.V.T. Doan-Nguyen, R.J. Gorte et al., Efficient removal of organic ligands from supported nanocrystals by fast thermal annealing enables catalytic studies on well-defined active phases. J. Am. Chem. Soc. 137(21), 6906–6911 (2015). https://doi.org/10.1021/jacs.5b03333
- D. Li, C. Wang, D. Tripkovic, S. Sun, N.M. Markovic et al., Surfactant removal for colloidal nanops from solution synthesis: the effect on catalytic performance. ACS Catal. 2(7), 1358–1362 (2012). https://doi.org/10.1021/cs300219j
- C. Susut, G.B. Chapman, G. Samjeské, M. Osawa, Y. Tong, An unexpected enhancement in methanol electro-oxidation on an ensemble of pt(111) nanofacets: a case of nanoscale single crystal ensemble electrocatalysis. PCCP 10(25), 3712–3721 (2008). https://doi.org/10.1039/B802708K
- C. Aliaga, J.Y. Park, Y. Yamada, H.S. Lee, C.-K. Tsung et al., Sum frequency generation and catalytic reaction studies of the removal of organic capping agents from Pt nanops by Uv−Ozone treatment. J. Phys. Chem. C 113(15), 6150–6155 (2009). https://doi.org/10.1021/jp8108946
- S. Shaw, X. Tian, T.F. Silva, J.M. Bobbitt, F. Naab et al., Selective removal of ligands from colloidal nanocrystal assemblies with non-oxidizing He plasmas. Chem. Mater. 30(17), 5961–5967 (2018). https://doi.org/10.1021/acs.chemmater.8b02095
- J.K. Nørskov, T. Bligaard, B. Hvolbæk, F. Abild-Pedersen, I. Chorkendorff et al., The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 37(10), 2163–2171 (2008). https://doi.org/10.1039/B800260F
- T. Bligaard, J.K. Nørskov, S. Dahl, J. Matthiesen, C.H. Christensen et al., The brønsted–evans–polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224(1), 206–217 (2004). https://doi.org/10.1016/j.jcat.2004.02.034
- M. Dion, H. Rydberg, E. Schröder, D.C. Langreth, B.I. Lundqvist, van der Waals density functional for general geometries. Phys. Rev. Lett. 92(24), 246401 (2004). https://doi.org/10.1103/PhysRevLett.92.246401
- S. Linic, M.A. Barteau, Construction of a reaction coordinate and a microkinetic model for ethylene epoxidation on silver from DFT calculations and surface science experiments. J. Catal. 214(2), 200–212 (2003). https://doi.org/10.1016/S0021-9517(02)00156-2
- Z. Liu, Z. Zhao, B. Peng, X. Duan, Y. Huang, Beyond extended surfaces: understanding the oxygen reduction reaction on nanocatalysts. J. Am. Chem. Soc. 142(42), 17812–17827 (2020). https://doi.org/10.1021/jacs.0c07696
- Y. Chen, T. Cheng, W.A.G. Iii, Atomistic explanation of the dramatically improved oxygen reduction reaction of jagged platinum nanowires 50 times better than Pt. J. Am. Chem. Soc. 142(19), 8625–8632 (2020). https://doi.org/10.1021/jacs.9b13218
- F.T. Wagner, B. Lakshmanan, M.F. Mathias, Electrochemistry and the future of the automobile. J. Phys. Chem. Lett. 1(14), 2204–2219 (2010). https://doi.org/10.1021/jz100553m
- Y. Shi, Z. Lyu, M. Zhao, R. Chen, Q.N. Nguyen et al., Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 121(2), 649–735 (2021). https://doi.org/10.1021/acs.chemrev.0c00454
- R.G. Chaudhuri, S. Paria, Core/shell nanops: classes. Properties. Synthesis mechanisms, characterization, and applications. Chem. Rev. 112(4), 2373–2433 (2012). https://doi.org/10.1021/cr100449n
- Z. Zhao, C. Chen, Z. Liu, J. Huang, M. Wu et al., Pt-based nanocrystal for electrocatalytic oxygen reduction. Adv. Mater. 31(31), 1808115 (2019). https://doi.org/10.1002/adma.201808115
- H. Yang, J. Zhang, K. Sun, S. Zou, J. Fang, Enhancing by weakening: electrooxidation of methanol on Pt3Co and Pt nanocubes. Angew. Chem. Int. Ed. 49(38), 6848–6851 (2010). https://doi.org/10.1002/anie.201002888
- Z. Quan, Y. Wang, J. Fang, High-index faceted noble metal nanocrystals. Acc. Chem. Res. 46(2), 191–202 (2013). https://doi.org/10.1021/ar200293n
- C. Shen, X. Li, Y. Wei, Z. Cao, H. Li et al., PtCo-excavated rhombic dodecahedral nanocrystals for efficient electrocatalysis. Nanoscale Adv. 2(10), 4881–4886 (2020). https://doi.org/10.1039/D0NA00717J
- H.-S. Chen, T.M. Benedetti, J. Lian, S. Cheong, P.B. O’Mara et al., Role of the secondary metal in ordered and disordered Pt–M intermetallic nanops: an example of Pt3Sn nanocubes for the electrocatalytic methanol oxidation. ACS Catal. 11(4), 2235–2243 (2021). https://doi.org/10.1021/acscatal.0c05370
- L. Wei, Y.-J. Mao, F. Liu, T. Sheng, Y.-S. Wei et al., Concave cubic Pt–Sm alloy nanocrystals with high-index facets and enhanced electrocatalytic ethanol oxidation. ACS Appl. Energy Mater. 2(10), 7204–7210 (2019). https://doi.org/10.1021/acsaem.9b01168
- X. Wu, Y. Jiang, Y. Yan, X. Li, S. Luo et al., Tuning surface structure of Pd3Pb/PtnPb nanocrystals for boosting the methanol oxidation reaction. Adv. Sci. 6(24), 1902249 (2019). https://doi.org/10.1002/advs.201902249
- N. Erini, S. Rudi, V. Beermann, P. Krause, R. Yang et al., Exceptional activity of a Pt–Rh–Ni ternary nanostructured catalyst for the electrochemical oxidation of ethanol. ChemElectroChem 2(6), 903–908 (2015). https://doi.org/10.1002/celc.201402390
- N. Erini, V. Beermann, M. Gocyla, M. Gliech, M. Heggen et al., The effect of surface site ensembles on the activity and selectivity of ethanol electrooxidation by octahedral PtNiRh nanops. Angew. Chem. Int. Ed. 56(23), 6533–6538 (2017). https://doi.org/10.1002/anie.201702332
- T. Zhang, Single-atom catalysis: far beyond the matter of metal dispersion. Nano Lett. 21(23), 9835–9837 (2021). https://doi.org/10.1021/acs.nanolett.1c02681
- R.T. Hannagan, G. Giannakakis, M. Flytzani-Stephanopoulos, E.C.H. Sykes, Single-atom alloy catalysis. Chem. Rev. 120(21), 12044–12088 (2020). https://doi.org/10.1021/acs.chemrev.0c00078
- Q. Chang, Y. Hong, H.J. Lee, J.H. Lee, D. Ologunagba et al., Achieving complete electrooxidation of ethanol by single atomic Rh decoration of Pt nanocubes. Proc. Natl. Acad. Sci. 119(11), e2112109119 (2022). https://doi.org/10.1073/pnas.2112109119
- L. Su, D. Gong, Y. Jin, D. Wu, W. Luo, Recent advances in alkaline hydrogen oxidation reaction. J. Energy Chem. 66, 107–122 (2022). https://doi.org/10.1016/j.jechem.2021.07.015
- J. Durst, A. Siebel, C. Simon, F. Hasché, J. Herranz et al., New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7(7), 2255–2260 (2014). https://doi.org/10.1039/C4EE00440J
- D. Strmcnik, M. Uchimura, C. Wang, R. Subbaraman, N. Danilovic et al., Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5(4), 300–306 (2013). https://doi.org/10.1038/nchem.1574
- L. An, X. Zhao, T. Zhao, D. Wang, Atomic-level insight into reasonable design of metal-based catalysts for hydrogen oxidation in alkaline electrolytes. Energy Environ. Sci. 14(5), 2620–2638 (2021). https://doi.org/10.1039/D0EE03609A
- T.J. Schmidt, P.N. Ross, N.M. Markovic, Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolytes: part 2. the hydrogen evolution/oxidation reaction. J. Electroanal. Chem. 524–525, 252–260 (2002). https://doi.org/10.1016/S0022-0728(02)00683-6
- Z.-Y. Zhou, Z.-Z. Huang, D.-J. Chen, Q. Wang, N. Tian et al., High-index faceted platinum nanocrystals supported on carbon black as highly efficient catalysts for ethanol electrooxidation. Angew. Chem. Int. Ed. 49(2), 411–414 (2010). https://doi.org/10.1002/anie.200905413
- N. Hoshi, Y. Asaumi, M. Nakamura, K. Mikita, R. Kajiwara, Structural effects on the hydrogen oxidation reaction on n(111)−(111) surfaces of platinum. J. Phys. Chem. C 113(39), 16843–16846 (2009). https://doi.org/10.1021/jp9076239
- H. Shan, W. Gao, Y. Xiong, F. Shi, Y. Yan et al., Nanoscale kinetics of asymmetrical corrosion in core-shell nanops. Nat. Commun. 9(1), 1–9 (2018). https://doi.org/10.1038/s41467-018-03372-z
- L. Chen, A. Leonardi, J. Chen, M. Cao, N. Li et al., Imaging the kinetics of anisotropic dissolution of bimetallic core-shell nanocubes using graphene liquid cells. Nat. Commun. 11(1), 3041 (2020). https://doi.org/10.1038/s41467-020-16645-3
- J.E. Evans, K.L. Jungjohann, N.D. Browning, I. Arslan, Controlled growth of nanops from solution with in situ liquid transmission electron microscopy. Nano Lett. 11(7), 2809–2813 (2011). https://doi.org/10.1021/nl201166k
- Z. Quan, Z. Luo, Y. Wang, H. Xu, C. Wang et al., Pressure-induced switching between amorphization and crystallization in PbTe nanops. Nano Lett. 13(8), 3729–3735 (2013). https://doi.org/10.1021/nl4016705
- V. Beermann, M.E. Holtz, E. Padgett, J.F. de Araujo, D.A. Muller et al., Real-time imaging of activation and degradation of carbon supported octahedral Pt–Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell stem. Energy Environ. Sci. 12(8), 2476–2485 (2019). https://doi.org/10.1039/C9EE01185D
- S. Rasouli, D. Myers, N. Kariuki, K. Higashida, N. Nakashima et al., Electrochemical degradation of Pt–Ni nanocatalysts: an identical location aberration-corrected scanning transmission electron microscopy study. Nano Lett. 19(1), 46–53 (2019). https://doi.org/10.1021/acs.nanolett.8b03022
- X.-L. Xi, M. Feng, L.-W. Zhang, Z.-R. Nie, Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. Int. J. Miner. Metall. Mater. 27(12), 1599–1617 (2020). https://doi.org/10.1007/s12613-020-2175-0
- S.-Q. Jiao, H.-D. Jiao, W.-L. Song, M.-Y. Wang, J.-G. Tu, A review on liquid metals as cathodes for molten salt/oxide electrolysis. Int. J. Miner. Metall. Mater. 27(12), 1588–1598 (2020). https://doi.org/10.1007/s12613-020-1971-x
References
K.D. Gilroy, A. Ruditskiy, H.-C. Peng, D. Qin, Y. Xia, Bimetallic nanocrystals: syntheses, properties, and applications. Chem. Rev. 116(18), 10414–10472 (2016). https://doi.org/10.1021/acs.chemrev.6b00211
M. Zhou, C. Li, J. Fang, Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications. Chem. Rev. 121(2), 736–795 (2021). https://doi.org/10.1021/acs.chemrev.0c00436
C. Li, S. Yan, J. Fang, Construction of lattice strain in bimetallic nanostructures and its effectiveness in electrochemical applications. Small 17(46), 2102244 (2021). https://doi.org/10.1002/smll.202102244
Y. Xia, Y. Xiong, B. Lim, S.E. Skrabalak, Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48(1), 60–103 (2009). https://doi.org/10.1002/anie.200802248
N.S. Porter, H. Wu, Z. Quan, J. Fang, Shape-control and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc. Chem. Res. 46(8), 1867–1877 (2013). https://doi.org/10.1021/ar3002238
A.R. Tao, S. Habas, P. Yang, Shape control of colloidal metal nanocrystals. Small 4(3), 310–325 (2008). https://doi.org/10.1002/smll.200701295
Y. Kang, C.B. Murray, Synthesis and electrocatalytic properties of cubic Mn−Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 132(22), 7568–7569 (2010). https://doi.org/10.1021/ja100705j
Y. Kang, J.B. Pyo, X. Ye, T.R. Gordon, C.B. Murray, Synthesis, shape control, and methanol electro-oxidation properties of Pt-Zn alloy and Pt3Zn intermetallic nanocrystals. ACS Nano 6(6), 5642–5647 (2012). https://doi.org/10.1021/nn301583g
B. Arumugam, T. Tamaki, T. Yamaguchi, Beneficial role of copper in the enhancement of durability of ordered intermetallic PtFeCu catalyst for electrocatalytic oxygen reduction. ACS Appl. Mater. Interfaces 7(30), 16311–16321 (2015). https://doi.org/10.1021/acsami.5b03137
D. Wang, H.L. Xin, R. Hovden, H. Wang, Y. Yu et al., Structurally ordered intermetallic platinum-cobalt core-shell nanops with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12(1), 81–87 (2013). https://doi.org/10.1038/nmat3458
J. Li, S. Sun, Intermetallic nanops: synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 52(7), 2015–2025 (2019). https://doi.org/10.1021/acs.accounts.9b00172
H.S. Chen, T.M. Benedetti, V.R. Goncales, N.M. Bedford, R.W.J. Scott et al., Preserving the exposed facets of Pt3Sn intermetallic nanocubes during an order to disorder transition allows the elucidation of the effect of the degree of alloy ordering on electrocatalysis. J. Am. Chem. Soc. 142(6), 3231–3239 (2020). https://doi.org/10.1021/jacs.9b13313
V.R. Stamenkovic, B. Fowler, B.S. Mun, G. Wang, P.N. Ross et al., Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315(5811), 493–497 (2007). https://doi.org/10.1126/science.1135941
V.R. Stamenkovic, B.S. Mun, M. Arenz, K.J.J. Mayrhofer, C.A. Lucas et al., Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6(3), 241–247 (2007). https://doi.org/10.1038/nmat1840
J. Greeley, I.E.L. Stephens, A.S. Bondarenko, T.P. Johansson, H.A. Hansen et al., Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1(7), 552–556 (2009). https://doi.org/10.1038/nchem.367
J. Wu, A. Gross, H. Yang, Shape and composition-controlled platinum alloy nanocrystals using carbon monoxide as reducing agent. Nano Lett. 11(2), 798–802 (2011). https://doi.org/10.1021/nl104094p
J. Zhang, H. Yang, J. Fang, S. Zou, Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 10(2), 638–644 (2010). https://doi.org/10.1021/nl903717z
Z. Ma, Z.P. Cano, A. Yu, Z. Chen, G. Jiang et al., Enhancing oxygen reduction activity of Pt-based electrocatalysts: from theoretical mechanisms to practical methods. Angew. Chem. Int. Ed. 59(42), 18334–18348 (2020). https://doi.org/10.1002/anie.202003654
F. Kong, Z. Ren, M.N. Banis, L. Du, X. Zhou et al., Active and stable Pt–Ni alloy octahedra catalyst for oxygen reduction via near-surface atomical engineering. ACS Catal. 10(7), 4205–4214 (2020). https://doi.org/10.1021/acscatal.9b05133
M. Nunez, J.L. Lansford, D.G. Vlachos, Optimization of the facet structure of transition-metal catalysts applied to the oxygen reduction reaction. Nat. Chem. 11(5), 449–456 (2019). https://doi.org/10.1038/s41557-019-0247-4
X. Huang, Z. Zhao, L. Cao, Y. Chen, E. Zhu et al., High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348(6240), 1230–1234 (2015). https://doi.org/10.1126/science.aaa8765
S.I. Choi, S. Xie, M. Shao, J.H. Odell, N. Lu et al., Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mgPt for the oxygen reduction reaction. Nano Lett. 13(7), 3420–3425 (2013). https://doi.org/10.1021/nl401881z
H. Frey, A. Beck, X. Huang, J.A. van Bokhoven, M.G. Willinger, Dynamic interplay between metal nanops and oxide support under redox conditions. Science 376(6596), 982–987 (2022). https://doi.org/10.1126/science.abm3371
Z. Luo, G. Zhao, H. Pan, W. Sun, Strong metal–support interaction in heterogeneous catalysts. Adv. Energy Mater. 12(37), 2201395 (2022). https://doi.org/10.1002/aenm.202201395
A. Shan, X. Teng, Y. Zhang, P. Zhang, Y. Xu et al., Interfacial electronic structure modulation of Pt-MoS2 heterostructure for enhancing electrocatalytic hydrogen evolution reaction. Nano Energy 94, 106913 (2022). https://doi.org/10.1016/j.nanoen.2021.106913
H. Mistry, A.S. Varela, S. Kühl, P. Strasser, B.R. Cuenya, Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1(4), 16009 (2016). https://doi.org/10.1038/natrevmats.2016.9
P. Yin, S.-C. Shen, L.-L. Zhang, X.-S. Zheng, M. Zuo et al., Ultra-high-temperature strong metal-support interactions in carbon-supported catalysts. Cell Rep. Phys. Sci. 3(8), 100984 (2022). https://doi.org/10.1016/j.xcrp.2022.100984
Y.T. Guntern, V. Okatenko, J. Pankhurst, S.B. Varandili, P. Iyengar et al., Colloidal nanocrystals as electrocatalysts with tunable activity and selectivity. ACS Catal. 11(3), 1248–1295 (2021). https://doi.org/10.1021/acscatal.0c04403
Y. Xia, X. Xia, H.-C. Peng, Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 137(25), 7947–7966 (2015). https://doi.org/10.1021/jacs.5b04641
C. Wang, C. Lin, L. Zhang, Z. Quan, K. Sun et al., Pt3Co concave nanocubes: synthesis, formation understanding, and enhanced catalytic activity toward hydrogenation of styrene. Chem. Eur. J. 20(6), 1753–1759 (2014). https://doi.org/10.1002/chem.201301724
J. Zhang, J. Fang, A general strategy for preparation of Pt 3d-transition metal (Co, Fe, Ni) nanocubes. J. Am. Chem. Soc. 131(51), 18543–18547 (2009). https://doi.org/10.1021/ja908245r
C. Wang, C. Lin, B. Zhao, L. Zhang, A. Kumbhar et al., High-indexed Pt3Fe nanocatalysts and their enhanced catalytic performance in dual organic reactions. ChemNanoMat. 1(5), 331–337 (2015). https://doi.org/10.1002/cnma.201500048
C. Li, J. Pan, L. Zhang, J. Fang, Colloidal synthesis of monodisperse trimetallic Pt–Fe–Ni nanocrystals and their enhanced electrochemical performances. Nanotechnology 34(7), 075401 (2023). https://doi.org/10.1088/1361-6528/aca337
Y. Kang, X. Ye, C.B. Murray, Size- and shape-selective synthesis of metal nanocrystals and nanowires using co as a reducing agent. Angew. Chem. Int. Ed. 49(35), 6156–6159 (2010). https://doi.org/10.1002/anie.201003383
M. Chen, J. Kim, J.P. Liu, H. Fan, S. Sun, Synthesis of FePt nanocubes and their oriented self-assembly. J. Am. Chem. Soc. 128(22), 7132–7133 (2006). https://doi.org/10.1021/ja061704x
S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Monodisperse FePt nanops and ferromagnetic FePt nanocrystal superlattices. Science 287(5460), 1989–1992 (2000). https://doi.org/10.1126/science.287.5460.1989
D. Xu, Z. Liu, H. Yang, Q. Liu, J. Zhang et al., Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum–copper nanocubes. Angew. Chem. Int. Ed. 48(23), 4217–4221 (2009). https://doi.org/10.1002/anie.200900293
G. Almeida, L. Goldoni, Q. Akkerman, Z. Dang, A.H. Khan et al., Role of acid-base equilibria in the size, shape, and phase control of cesium lead bromide nanocrystals. ACS Nano 12(2), 1704–1711 (2018). https://doi.org/10.1021/acsnano.7b08357
C. Chen, Y. Kang, Z. Huo, Z. Zhu, W. Huang et al., Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343(6177), 1339–1343 (2014). https://doi.org/10.1126/science.1249061
J. Zhang, H. Yang, B. Martens, Z. Luo, D. Xu et al., Pt–Cu nanoctahedra: synthesis and comparative study with nanocubes on their electrochemical catalytic performance. Chem. Sci. 3(11), 3302–3306 (2012). https://doi.org/10.1039/C2SC20514A
Y. Wang, J. He, C. Liu, W.H. Chong, H. Chen, Thermodynamics versus kinetics in nanosynthesis. Angew. Chem. Int. Ed. 54(7), 2022–2051 (2015). https://doi.org/10.1002/anie.201402986
N. Pradhan, D. Reifsnyder, R. Xie, J. Aldana, X. Peng, Surface ligand dynamics in growth of nanocrystals. J. Am. Chem. Soc. 129(30), 9500–9509 (2007). https://doi.org/10.1021/ja0725089
R. Costi, A.E. Saunders, U. Banin, Colloidal hybrid nanostructures: a new type of functional materials. Angew. Chem. Int. Ed. 49(29), 4878–4897 (2010). https://doi.org/10.1002/anie.200906010
Y. Wang, H.-C. Peng, J. Liu, C.Z. Huang, Y. Xia, Use of reduction rate as a quantitative knob for controlling the twin structure and shape of palladium nanocrystals. Nano Lett. 15(2), 1445–1450 (2015). https://doi.org/10.1021/acs.nanolett.5b00158
V.K. LaMer, R.H. Dinegar, Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 72(11), 4847–4854 (1950). https://doi.org/10.1021/ja01167a001
S. Xie, S.-I. Choi, N. Lu, L.T. Roling, J.A. Herron et al., Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 14(6), 3570–3576 (2014). https://doi.org/10.1021/nl501205j
G. Wulff, On the question of the rate of growth and dissolution of crystal surfaces. Z. Kristallogr. Mineral. 34, 449–530 (1901)
P.J.M. Smeets, K.R. Cho, R.G.E. Kempen, N.A.J.M. Sommerdijk, J.J. De Yoreo, Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat. Mater. 14(4), 394–399 (2015). https://doi.org/10.1038/nmat4193
H.G. Liao, D. Zherebetskyy, H. Xin, C. Czarnik, P. Ercius et al., Facet development during platinum nanocube growth. Science 345(6199), 916–919 (2014). https://doi.org/10.1126/science.1253149
D. Li, M.H. Nielsen, J.R.I. Lee, C. Frandsen, J.F. Banfield et al., Direction-specific interactions control crystal growth by oriented attachment. Science 336(6084), 1014–1018 (2012). https://doi.org/10.1126/science.1219643
H. Zheng, R.K. Smith, Y.-W. Jun, C. Kisielowski, U. Dahmen et al., Observation of single colloidal platinum nanocrystal growth trajectories. Science 324(5932), 1309–1312 (2009). https://doi.org/10.1126/science.1172104
P.L. Hansen, J.B. Wagner, S. Helveg, J.R. Rostrup-Nielsen, B.S. Clausen et al., Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295(5562), 2053–2055 (2002). https://doi.org/10.1126/science.1069325
H.-G. Liao, L. Cui, S. Whitelam, H. Zheng, Real-time imaging of Pt3Fe nanorod growth in solution. Science 336(6084), 1011–1014 (2012). https://doi.org/10.1126/science.1219185
Z. Zeng, W. Zheng, H. Zheng, Visualization of colloidal nanocrystal formation and electrode–electrolyte interfaces in liquids using TEM. Acc. Chem. Res. 50(8), 1808–1817 (2017). https://doi.org/10.1021/acs.accounts.7b00161
X. Ye, M.R. Jones, L.B. Frechette, Q. Chen, A.S. Powers et al., Single-p mapping of nonequilibrium nanocrystal transformations. Science 354(6314), 874–877 (2016). https://doi.org/10.1126/science.aah4434
R. Long, S. Zhou, B.J. Wiley, Y. Xiong, Oxidative etching for controlled synthesis of metal nanocrystals: atomic addition and subtraction. Chem. Soc. Rev. 43(17), 6288–6310 (2014). https://doi.org/10.1039/C4CS00136B
Y. Jiang, G. Zhu, F. Lin, H. Zhang, C. Jin et al., In situ study of oxidative etching of palladium nanocrystals by liquid cell electron microscopy. Nano Lett. 14(7), 3761–3765 (2014). https://doi.org/10.1021/nl500670q
Q. Zheng, J. Shangguan, X. Li, Q. Zhang, K.C. Bustillo et al., Observation of surface ligands-controlled etching of palladium nanocrystals. Nano Lett. 21(15), 6640–6647 (2021). https://doi.org/10.1021/acs.nanolett.1c02104
H.-D. Yu, M.D. Regulacio, E. Ye, M.-Y. Han, Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 42(14), 6006–6018 (2013). https://doi.org/10.1039/C3CS60113G
M. Azharuddin, G.H. Zhu, D. Das, E. Ozgur, L. Uzun et al., A repertoire of biomedical applications of noble metal nanops. Chem. Commun. 55(49), 6964–6996 (2019). https://doi.org/10.1039/C9CC01741K
B.C. Steele, A. Heinzel, Materials for fuel-cell technologies. Nature 414(6861), 345–352 (2001). https://doi.org/10.1038/35104620
J. Chen, P. Mela, M. Möller, M.C. Lensen, Microcontact deprinting: a technique to pattern gold nanops. ACS Nano 3(6), 1451–1456 (2009). https://doi.org/10.1021/nn9002924
G. Walters, I.P. Parkin, The incorporation of noble metal nanops into host matrix thin films: synthesis, characterisation and applications. J. Mater. Chem. 19(5), 574–590 (2009). https://doi.org/10.1039/B809646E
L. Wang, D. Ma, C. Guo, X. Jiang, M. Li et al., CsPbBr 3 nanocrystals prepared by high energy ball milling in one-step and structural transformation from CsPbBr3 to CsPb2Br5. Appl. Surf. Sci. 543, 148782 (2021). https://doi.org/10.1016/j.apsusc.2020.148782
Y. Wang, Y. Xia, Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 4(10), 2047–2050 (2004). https://doi.org/10.1021/nl048689j
S.E. Skrabalak, J. Chen, Y. Sun, X. Lu, L. Au et al., Gold nanocages: synthesis, properties, and applications. Acc. Chem. Res. 41(12), 1587–1595 (2008). https://doi.org/10.1021/ar800018v
C. Wang, L. Zhang, H. Yang, J. Pan, J. Liu et al., High-indexed Pt3Ni alloy tetrahexahedral nanoframes evolved through preferential CO etching. Nano Lett. 17(4), 2204–2210 (2017). https://doi.org/10.1021/acs.nanolett.6b04731
L. Gan, M. Heggen, S. Rudi, P. Strasser, Core–shell compositional fine structures of dealloyed PtxNi1–x nanops and their impact on oxygen reduction catalysis. Nano Lett. 12(10), 5423–5430 (2012). https://doi.org/10.1021/nl302995z
M. Oezaslan, M. Heggen, P. Strasser, Size-dependent morphology of dealloyed bimetallic catalysts: linking the nano to the macro scale. J. Am. Chem. Soc. 134(1), 514–524 (2012). https://doi.org/10.1021/ja2088162
L. Gan, M. Heggen, R. O’Malley, B. Theobald, P. Strasser, Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 13(3), 1131–1138 (2013). https://doi.org/10.1021/nl304488q
Y.N. Tan, J.Y. Lee, D.I.C. Wang, Uncovering the design rules for peptide synthesis of metal nanops. J. Am. Chem. Soc. 132(16), 5677–5686 (2010). https://doi.org/10.1021/ja907454f
C.B.M.C.R. Kagan, M.G. Bawendi, Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 30(1), 545–610 (2000). https://doi.org/10.1146/annurev.matsci.30.1.545
Y. Wu, D. Wang, Z. Niu, P. Chen, G. Zhou et al., A strategy for designing a concave Pt-Ni alloy through controllable chemical etching. Angew. Chem. Int. Ed. 51(50), 12524–12528 (2012). https://doi.org/10.1002/anie.201207491
J. Gu, Y.-W. Zhang, F. Tao, Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 41(24), 8050–8065 (2012). https://doi.org/10.1039/C2CS35184F
Y. Wang, Z. Sun, A. Kumbhar, Z. Luo, C. Wang et al., Is CO adequate to facilitate the formation of Pt3M (M = Fe, Ni and Co) nanocubes? Chem. Commun. 49(38), 3955–3957 (2013). https://doi.org/10.1039/C3CC41424H
C.-K. Tsung, J.N. Kuhn, W. Huang, C. Aliaga, L.-I. Hung et al., Sub-10 nm platinum nanocrystals with size and shape control: catalytic study for ethylene and pyrrole hydrogenation. J. Am. Chem. Soc. 131(16), 5816–5822 (2009). https://doi.org/10.1021/ja809936n
J. Qian, M. Shen, S. Zhou, C.-T. Lee, M. Zhao et al., Synthesis of Pt nanocrystals with different shapes using the same protocol to optimize their catalytic activity toward oxygen reduction. Mater. Today 21(8), 834–844 (2018). https://doi.org/10.1016/j.mattod.2018.08.005
W. Zhu, A.X. Yin, Y.W. Zhang, C.H. Yan, Highly shape-selective synthesis of monodispersed fivefold twinned platinum nanodecahedrons and nanoicosahedrons. Chem. Eur. J. 18(39), 12222–12226 (2012). https://doi.org/10.1002/chem.201201099
N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding, Z.L. Wang, Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316(5825), 732–735 (2007). https://doi.org/10.1126/science.1140484
L. Huang, M. Liu, H. Lin, Y. Xu, J. Wu et al., Shape regulation of high-index facet nanops by dealloying. Science 365(6458), 1159–1163 (2019). https://doi.org/10.1126/science.aax5843
Y. Kang, J.B. Pyo, X. Ye, R.E. Diaz, T.R. Gordon et al., Shape-controlled synthesis of Pt nanocrystals: the role of metal carbonyls. ACS Nano 7(1), 645–653 (2013). https://doi.org/10.1021/nn3048439
C. Wang, H. Daimon, Y. Lee, J. Kim, S. Sun, Synthesis of monodisperse Pt nanocubes and their enhanced catalysis for oxygen reduction. J. Am. Chem. Soc. 129(22), 6974–6975 (2007). https://doi.org/10.1021/ja070440r
J. Ren, R.D. Tilley, Shape-controlled growth of platinum nanops. Small 3(9), 1508–1512 (2007). https://doi.org/10.1002/smll.200700135
C.-T. Lee, X. Yang, M. Vara, K.D. Gilroy, Y. Xia, Water-based synthesis of sub-10 nm Pt octahedra and their performance towards the oxygen reduction reaction. ChemNanoMat 3(12), 879–884 (2017). https://doi.org/10.1002/cnma.201700189
W. Zhou, J. Wu, H. Yang, Highly uniform platinum icosahedra made by hot injection-assisted grails method. Nano Lett. 13(6), 2870–2874 (2013). https://doi.org/10.1021/nl401214d
J. Zhang, Z. Luo, B. Martens, Z. Quan, A. Kumbhar et al., Reversible kirkwood–alder transition observed in Pt3Cu2 nanoctahedron assemblies under controlled solvent annealing/drying conditions. J. Am. Chem. Soc. 134(34), 14043–14049 (2012). https://doi.org/10.1021/ja304108n
J. Ding, L. Bu, S. Guo, Z. Zhao, E. Zhu et al., Morphology and phase controlled construction of Pt–Ni nanostructures for efficient electrocatalysis. Nano Lett. 16(4), 2762–2767 (2016). https://doi.org/10.1021/acs.nanolett.6b00471
S. Chen, M. Li, M. Gao, J. Jin, M.A. van Spronsen et al., High-performance Pt–Co nanoframes for fuel-cell electrocatalysis. Nano Lett. 20(3), 1974–1979 (2020). https://doi.org/10.1021/acs.nanolett.9b05251
C. Xie, Z. Niu, D. Kim, M. Li, P. Yang, Surface and interface control in nanop catalysis. Chem. Rev. 120(2), 1184–1249 (2020). https://doi.org/10.1021/acs.chemrev.9b00220
J.R. Greer, Nanoframe catalysts. Science 343(6177), 1319–1320 (2014). https://doi.org/10.1126/science.1251865
Q. Chen, Z. Cao, G. Du, Q. Kuang, J. Huang et al., Excavated octahedral Pt-Co alloy nanocrystals built with ultrathin nanosheets as superior multifunctional electrocatalysts for energy conversion applications. Nano Energy 39, 582–589 (2017). https://doi.org/10.1016/j.nanoen.2017.07.041
C. Cui, L. Gan, M. Heggen, S. Rudi, P. Strasser, Compositional segregation in shaped Pt alloy nanops and their structural behaviour during electrocatalysis. Nat. Mater. 12(8), 765–771 (2013). https://doi.org/10.1038/nmat3668
S.E. Habas, H. Lee, V. Radmilovic, G.A. Somorjai, P. Yang, Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 6(9), 692–697 (2007). https://doi.org/10.1038/nmat1957
F.-R. Fan, D.-Y. Liu, Y.-F. Wu, S. Duan, Z.-X. Xie et al., Epitaxial growth of heterogeneous metal nanocrystals: from gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 130(22), 6949–6951 (2008). https://doi.org/10.1021/ja801566d
T. Lv, X. Yang, Y. Zheng, H. Huang, L. Zhang et al., Controlling the growth of Au on icosahedral seeds of Pd by manipulating the reduction kinetics. J. Phys. Chem. C 120(37), 20768–20774 (2016). https://doi.org/10.1021/acs.jpcc.6b02022
M. Yang, W. Wang, K.D. Gilroy, Y. Xia, Controlling the deposition of Pd on au nanocages: outer surface only versus both outer and inner surfaces. Nano Lett. 17(9), 5682–5687 (2017). https://doi.org/10.1021/acs.nanolett.7b02578
L. Zhang, L.T. Roling, X. Wang, M. Vara, M. Chi et al., Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349(6246), 412–416 (2015). https://doi.org/10.1126/science.aab0801
J. Park, L. Zhang, S.-I. Choi, L.T. Roling, N. Lu et al., Atomic layer-by-layer deposition of platinum on palladium octahedra for enhanced catalysts toward the oxygen reduction reaction. ACS Nano 9(3), 2635–2647 (2015). https://doi.org/10.1021/nn506387w
H. Zhang, M. Jin, J. Wang, W. Li, P.H.C. Camargo et al., Synthesis of Pd−Pt bimetallic nanocrystals with a concave structure through a bromide-induced galvanic replacement reaction. J. Am. Chem. Soc. 133(15), 6078–6089 (2011). https://doi.org/10.1021/ja201156s
X. Wang, M. Vara, M. Luo, H. Huang, A. Ruditskiy et al., Pd@Pt core–shell concave decahedra: a class of catalysts for the oxygen reduction reaction with enhanced activity and durability. J. Am. Chem. Soc. 137(47), 15036–15042 (2015). https://doi.org/10.1021/jacs.5b10059
X. Wang, S.-I. Choi, L.T. Roling, M. Luo, C. Ma et al., Palladium–platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 6, 7594 (2015). https://doi.org/10.1038/ncomms8594
X. Wang, L. Figueroa-Cosme, X. Yang, M. Luo, J. Liu et al., Pt-based icosahedral nanocages: using a combination of 111 facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 16(2), 1467–1471 (2016). https://doi.org/10.1021/acs.nanolett.5b05140
D.S. He, D. He, J. Wang, Y. Lin, P. Yin et al., Ultrathin icosahedral Pt-enriched nanocage with excellent oxygen reduction reaction activity. J. Am. Chem. Soc. 138(5), 1494–1497 (2016). https://doi.org/10.1021/jacs.5b12530
B.Y. Xia, H.B. Wu, X. Wang, X.W. Lou, One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 134(34), 13934–13937 (2012). https://doi.org/10.1021/ja3051662
X. Tian, X. Zhao, Y.-Q. Su, L. Wang, H. Wang et al., Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366(6467), 850–856 (2019). https://doi.org/10.1126/science.aaw7493
F. Saleem, Z. Zhang, X. Cui, Y. Gong, B. Chen et al., Elemental segregation in multimetallic core–shell nanoplates. J. Am. Chem. Soc. 141(37), 14496–14500 (2019). https://doi.org/10.1021/jacs.9b05197
S. Zhang, Y. Hao, D. Su, V.V.T. Doan-Nguyen, Y. Wu et al., Monodisperse core/shell Ni/FePt nanops and their conversion to Ni/Pt to catalyze oxygen reduction. J. Am. Chem. Soc. 136(45), 15921–15924 (2014). https://doi.org/10.1021/ja5099066
C. Li, X. Chen, L. Zhang, S. Yan, A. Sharma et al., Synthesis of core@shell Cu-Ni@Pt-Cu nano-octahedra and their improved MOR activity. Angew. Chem. Int. Ed. 60(14), 7675–7680 (2021). https://doi.org/10.1002/anie.202014144
G. Liu, W. Zhou, Y. Ji, B. Chen, G. Fu et al., Hydrogen-intercalation-induced lattice expansion of Pd@Pt core–shell nanops for highly efficient electrocatalytic alcohol oxidation. J. Am. Chem. Soc. 143(29), 11262–11270 (2021). https://doi.org/10.1021/jacs.1c05856
M. Vara, X. Wang, J. Howe, M. Chi, Y. Xia, Understanding the stability of Pt-based nanocages under thermal stress using in situ electron microscopy. ChemNanoMat 4(1), 112–117 (2018). https://doi.org/10.1002/cnma.201700298
J.T.L. Gamler, H.M. Ashberry, S.E. Skrabalak, K.M. Koczkur, Random alloyed versus intermetallic nanops: a comparison of electrocatalytic performance. Adv. Mater. (2018). https://doi.org/10.1002/adma.201801563
J. Li, Z. Xi, Y.-T. Pan, J.S. Spendelow, P.N. Duchesne et al., Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanops for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 140(8), 2926–2932 (2018). https://doi.org/10.1021/jacs.7b12829
Q. Li, L. Wu, G. Wu, D. Su, H. Lv et al., New approach to fully ordered fct-FePt nanops for much enhanced electrocatalysis in acid. Nano Lett. 15(4), 2468–2473 (2015). https://doi.org/10.1021/acs.nanolett.5b00320
M. Xie, Z. Lyu, R. Chen, M. Shen, Z. Cao et al., Pt-Co@Pt octahedral nanocrystals: enhancing their activity and durability toward oxygen reduction with an intermetallic core and an ultrathin shell. J. Am. Chem. Soc. 143(22), 8509–8518 (2021). https://doi.org/10.1021/jacs.1c04160
J. Li, S. Sharma, K. Wei, Z. Chen, D. Morris et al., Anisotropic strain tuning of L10 ternary nanops for oxygen reduction. J. Am. Chem. Soc. 142(45), 19209–19216 (2020). https://doi.org/10.1021/jacs.0c08962
J. Li, S. Sharma, X. Liu, Y.-T. Pan, J.S. Spendelow et al., Hard-magnet L10-CoPt nanops advance fuel cell catalysis. Joule. 3(1), 124–135 (2019). https://doi.org/10.1016/j.joule.2018.09.016
X. Chen, S. Zhang, C. Li, Z. Liu, X. Sun et al., Composition-dependent ordering transformations in Pt–Fe nanoalloys. Proc. Natl. Acad. Sci. 119(14), e2117899119 (2022). https://doi.org/10.1073/pnas.2117899119
Y. Qin, M. Luo, Y. Sun, C. Li, B. Huang et al., Intermetallic hcp-PtBi/fcc-Pt core/shell nanoplates enable efficient bifunctional oxygen reduction and methanol oxidation electrocatalysis. ACS Catal. 8(6), 5581–5590 (2018). https://doi.org/10.1021/acscatal.7b04406
Q. Feng, S. Zhao, D. He, S. Tian, L. Gu et al., Strain engineering to enhance the electrooxidation performance of atomic-layer pt on intermetallic Pt3Ga. J. Am. Chem. Soc. 140(8), 2773–2776 (2018). https://doi.org/10.1021/jacs.7b13612
L. Bu, N. Zhang, S. Guo, X. Zhang, J. Li et al., Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354(6318), 1410–1414 (2016). https://doi.org/10.1126/science.aah6133
S. Maksimuk, S. Yang, Z. Peng, H. Yang, Synthesis and characterization of ordered intermetallic PtPb nanorods. J. Am. Chem. Soc. 129(28), 8684–8685 (2007). https://doi.org/10.1021/ja071980r
H. Rong, J. Mao, P. Xin, D. He, Y. Chen et al., Kinetically controlling surface structure to construct defect-rich intermetallic nanocrystals: effective and stable catalysts. Adv. Mater. 28(13), 2540–2546 (2016). https://doi.org/10.1002/adma.201504831
H. Yang, Y. Tang, S. Zou, Electrochemical removal of surfactants from Pt nanocubes. Electrochem. Commun. 38, 134–137 (2014). https://doi.org/10.1016/j.elecom.2013.11.019
P. Godbold, G. Johnson, A.D. Obi, R. Brown, S. Hwang et al., Surfactant removal for colloidal nanocrystal catalysts mediated by n-heterocyclic carbenes. J. Am. Chem. Soc. 143(7), 2644–2648 (2021). https://doi.org/10.1021/jacs.0c12278
W.F. Fu, Y. Shi, L. Wang, M.M. Shi, H.Y. Li et al., A green, low-cost, and highly effective strategy to enhance the performance of hybrid solar cells: post-deposition ligand exchange by acetic acid. Sol. Energy Mater. Sol. Cells. 117, 329–335 (2013). https://doi.org/10.1016/j.solmat.2013.06.042
Z. Zhang, M. Chi, G.M. Veith, P. Zhang, D.A. Lutterman et al., Rational design of Bi nanops for efficient electrochemical CO2 reduction: the elucidation of size and surface condition effects. ACS Catal. 6(9), 6255–6264 (2016). https://doi.org/10.1021/acscatal.6b01297
M. Zhou, H. Wang, L. Zhang, C. Li, A. Kumbhar et al., Facet impact of CuMn2O4 spinel nanocatalysts on enhancement of the oxygen reduction reaction in alkaline media. ACS Catal. 12(21), 13663–13670 (2022). https://doi.org/10.1021/acscatal.2c03275
R. Latsuzbaia, E. Negro, G. Koper, Synthesis, stabilization and activation of pt nanops for PEMFC applications. Fuel Cells 15(4), 628–638 (2015). https://doi.org/10.1002/fuce.201500023
M. Cargnello, C. Chen, B.T. Diroll, V.V.T. Doan-Nguyen, R.J. Gorte et al., Efficient removal of organic ligands from supported nanocrystals by fast thermal annealing enables catalytic studies on well-defined active phases. J. Am. Chem. Soc. 137(21), 6906–6911 (2015). https://doi.org/10.1021/jacs.5b03333
D. Li, C. Wang, D. Tripkovic, S. Sun, N.M. Markovic et al., Surfactant removal for colloidal nanops from solution synthesis: the effect on catalytic performance. ACS Catal. 2(7), 1358–1362 (2012). https://doi.org/10.1021/cs300219j
C. Susut, G.B. Chapman, G. Samjeské, M. Osawa, Y. Tong, An unexpected enhancement in methanol electro-oxidation on an ensemble of pt(111) nanofacets: a case of nanoscale single crystal ensemble electrocatalysis. PCCP 10(25), 3712–3721 (2008). https://doi.org/10.1039/B802708K
C. Aliaga, J.Y. Park, Y. Yamada, H.S. Lee, C.-K. Tsung et al., Sum frequency generation and catalytic reaction studies of the removal of organic capping agents from Pt nanops by Uv−Ozone treatment. J. Phys. Chem. C 113(15), 6150–6155 (2009). https://doi.org/10.1021/jp8108946
S. Shaw, X. Tian, T.F. Silva, J.M. Bobbitt, F. Naab et al., Selective removal of ligands from colloidal nanocrystal assemblies with non-oxidizing He plasmas. Chem. Mater. 30(17), 5961–5967 (2018). https://doi.org/10.1021/acs.chemmater.8b02095
J.K. Nørskov, T. Bligaard, B. Hvolbæk, F. Abild-Pedersen, I. Chorkendorff et al., The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 37(10), 2163–2171 (2008). https://doi.org/10.1039/B800260F
T. Bligaard, J.K. Nørskov, S. Dahl, J. Matthiesen, C.H. Christensen et al., The brønsted–evans–polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224(1), 206–217 (2004). https://doi.org/10.1016/j.jcat.2004.02.034
M. Dion, H. Rydberg, E. Schröder, D.C. Langreth, B.I. Lundqvist, van der Waals density functional for general geometries. Phys. Rev. Lett. 92(24), 246401 (2004). https://doi.org/10.1103/PhysRevLett.92.246401
S. Linic, M.A. Barteau, Construction of a reaction coordinate and a microkinetic model for ethylene epoxidation on silver from DFT calculations and surface science experiments. J. Catal. 214(2), 200–212 (2003). https://doi.org/10.1016/S0021-9517(02)00156-2
Z. Liu, Z. Zhao, B. Peng, X. Duan, Y. Huang, Beyond extended surfaces: understanding the oxygen reduction reaction on nanocatalysts. J. Am. Chem. Soc. 142(42), 17812–17827 (2020). https://doi.org/10.1021/jacs.0c07696
Y. Chen, T. Cheng, W.A.G. Iii, Atomistic explanation of the dramatically improved oxygen reduction reaction of jagged platinum nanowires 50 times better than Pt. J. Am. Chem. Soc. 142(19), 8625–8632 (2020). https://doi.org/10.1021/jacs.9b13218
F.T. Wagner, B. Lakshmanan, M.F. Mathias, Electrochemistry and the future of the automobile. J. Phys. Chem. Lett. 1(14), 2204–2219 (2010). https://doi.org/10.1021/jz100553m
Y. Shi, Z. Lyu, M. Zhao, R. Chen, Q.N. Nguyen et al., Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 121(2), 649–735 (2021). https://doi.org/10.1021/acs.chemrev.0c00454
R.G. Chaudhuri, S. Paria, Core/shell nanops: classes. Properties. Synthesis mechanisms, characterization, and applications. Chem. Rev. 112(4), 2373–2433 (2012). https://doi.org/10.1021/cr100449n
Z. Zhao, C. Chen, Z. Liu, J. Huang, M. Wu et al., Pt-based nanocrystal for electrocatalytic oxygen reduction. Adv. Mater. 31(31), 1808115 (2019). https://doi.org/10.1002/adma.201808115
H. Yang, J. Zhang, K. Sun, S. Zou, J. Fang, Enhancing by weakening: electrooxidation of methanol on Pt3Co and Pt nanocubes. Angew. Chem. Int. Ed. 49(38), 6848–6851 (2010). https://doi.org/10.1002/anie.201002888
Z. Quan, Y. Wang, J. Fang, High-index faceted noble metal nanocrystals. Acc. Chem. Res. 46(2), 191–202 (2013). https://doi.org/10.1021/ar200293n
C. Shen, X. Li, Y. Wei, Z. Cao, H. Li et al., PtCo-excavated rhombic dodecahedral nanocrystals for efficient electrocatalysis. Nanoscale Adv. 2(10), 4881–4886 (2020). https://doi.org/10.1039/D0NA00717J
H.-S. Chen, T.M. Benedetti, J. Lian, S. Cheong, P.B. O’Mara et al., Role of the secondary metal in ordered and disordered Pt–M intermetallic nanops: an example of Pt3Sn nanocubes for the electrocatalytic methanol oxidation. ACS Catal. 11(4), 2235–2243 (2021). https://doi.org/10.1021/acscatal.0c05370
L. Wei, Y.-J. Mao, F. Liu, T. Sheng, Y.-S. Wei et al., Concave cubic Pt–Sm alloy nanocrystals with high-index facets and enhanced electrocatalytic ethanol oxidation. ACS Appl. Energy Mater. 2(10), 7204–7210 (2019). https://doi.org/10.1021/acsaem.9b01168
X. Wu, Y. Jiang, Y. Yan, X. Li, S. Luo et al., Tuning surface structure of Pd3Pb/PtnPb nanocrystals for boosting the methanol oxidation reaction. Adv. Sci. 6(24), 1902249 (2019). https://doi.org/10.1002/advs.201902249
N. Erini, S. Rudi, V. Beermann, P. Krause, R. Yang et al., Exceptional activity of a Pt–Rh–Ni ternary nanostructured catalyst for the electrochemical oxidation of ethanol. ChemElectroChem 2(6), 903–908 (2015). https://doi.org/10.1002/celc.201402390
N. Erini, V. Beermann, M. Gocyla, M. Gliech, M. Heggen et al., The effect of surface site ensembles on the activity and selectivity of ethanol electrooxidation by octahedral PtNiRh nanops. Angew. Chem. Int. Ed. 56(23), 6533–6538 (2017). https://doi.org/10.1002/anie.201702332
T. Zhang, Single-atom catalysis: far beyond the matter of metal dispersion. Nano Lett. 21(23), 9835–9837 (2021). https://doi.org/10.1021/acs.nanolett.1c02681
R.T. Hannagan, G. Giannakakis, M. Flytzani-Stephanopoulos, E.C.H. Sykes, Single-atom alloy catalysis. Chem. Rev. 120(21), 12044–12088 (2020). https://doi.org/10.1021/acs.chemrev.0c00078
Q. Chang, Y. Hong, H.J. Lee, J.H. Lee, D. Ologunagba et al., Achieving complete electrooxidation of ethanol by single atomic Rh decoration of Pt nanocubes. Proc. Natl. Acad. Sci. 119(11), e2112109119 (2022). https://doi.org/10.1073/pnas.2112109119
L. Su, D. Gong, Y. Jin, D. Wu, W. Luo, Recent advances in alkaline hydrogen oxidation reaction. J. Energy Chem. 66, 107–122 (2022). https://doi.org/10.1016/j.jechem.2021.07.015
J. Durst, A. Siebel, C. Simon, F. Hasché, J. Herranz et al., New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7(7), 2255–2260 (2014). https://doi.org/10.1039/C4EE00440J
D. Strmcnik, M. Uchimura, C. Wang, R. Subbaraman, N. Danilovic et al., Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5(4), 300–306 (2013). https://doi.org/10.1038/nchem.1574
L. An, X. Zhao, T. Zhao, D. Wang, Atomic-level insight into reasonable design of metal-based catalysts for hydrogen oxidation in alkaline electrolytes. Energy Environ. Sci. 14(5), 2620–2638 (2021). https://doi.org/10.1039/D0EE03609A
T.J. Schmidt, P.N. Ross, N.M. Markovic, Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolytes: part 2. the hydrogen evolution/oxidation reaction. J. Electroanal. Chem. 524–525, 252–260 (2002). https://doi.org/10.1016/S0022-0728(02)00683-6
Z.-Y. Zhou, Z.-Z. Huang, D.-J. Chen, Q. Wang, N. Tian et al., High-index faceted platinum nanocrystals supported on carbon black as highly efficient catalysts for ethanol electrooxidation. Angew. Chem. Int. Ed. 49(2), 411–414 (2010). https://doi.org/10.1002/anie.200905413
N. Hoshi, Y. Asaumi, M. Nakamura, K. Mikita, R. Kajiwara, Structural effects on the hydrogen oxidation reaction on n(111)−(111) surfaces of platinum. J. Phys. Chem. C 113(39), 16843–16846 (2009). https://doi.org/10.1021/jp9076239
H. Shan, W. Gao, Y. Xiong, F. Shi, Y. Yan et al., Nanoscale kinetics of asymmetrical corrosion in core-shell nanops. Nat. Commun. 9(1), 1–9 (2018). https://doi.org/10.1038/s41467-018-03372-z
L. Chen, A. Leonardi, J. Chen, M. Cao, N. Li et al., Imaging the kinetics of anisotropic dissolution of bimetallic core-shell nanocubes using graphene liquid cells. Nat. Commun. 11(1), 3041 (2020). https://doi.org/10.1038/s41467-020-16645-3
J.E. Evans, K.L. Jungjohann, N.D. Browning, I. Arslan, Controlled growth of nanops from solution with in situ liquid transmission electron microscopy. Nano Lett. 11(7), 2809–2813 (2011). https://doi.org/10.1021/nl201166k
Z. Quan, Z. Luo, Y. Wang, H. Xu, C. Wang et al., Pressure-induced switching between amorphization and crystallization in PbTe nanops. Nano Lett. 13(8), 3729–3735 (2013). https://doi.org/10.1021/nl4016705
V. Beermann, M.E. Holtz, E. Padgett, J.F. de Araujo, D.A. Muller et al., Real-time imaging of activation and degradation of carbon supported octahedral Pt–Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell stem. Energy Environ. Sci. 12(8), 2476–2485 (2019). https://doi.org/10.1039/C9EE01185D
S. Rasouli, D. Myers, N. Kariuki, K. Higashida, N. Nakashima et al., Electrochemical degradation of Pt–Ni nanocatalysts: an identical location aberration-corrected scanning transmission electron microscopy study. Nano Lett. 19(1), 46–53 (2019). https://doi.org/10.1021/acs.nanolett.8b03022
X.-L. Xi, M. Feng, L.-W. Zhang, Z.-R. Nie, Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. Int. J. Miner. Metall. Mater. 27(12), 1599–1617 (2020). https://doi.org/10.1007/s12613-020-2175-0
S.-Q. Jiao, H.-D. Jiao, W.-L. Song, M.-Y. Wang, J.-G. Tu, A review on liquid metals as cathodes for molten salt/oxide electrolysis. Int. J. Miner. Metall. Mater. 27(12), 1588–1598 (2020). https://doi.org/10.1007/s12613-020-1971-x