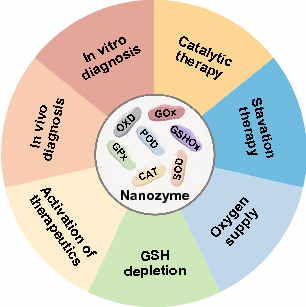
Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy
Corresponding Author: Yanli Zhao
Nano-Micro Letters,
Vol. 14 (2022), Article Number: 95
Abstract
Natural enzymes usually suffer from high production cost, ease of denaturation and inactivation, and low yield, making them difficult to be broadly applicable. As an emerging type of artificial enzyme, nanozymes that combine the characteristics of nanomaterials and enzymes are promising alternatives. On the one hand, nanozymes have high enzyme-like catalytic activities to regulate biochemical reactions. On the other hand, nanozymes also inherit the properties of nanomaterials, which can ameliorate the shortcomings of natural enzymes and serve as versatile platforms for diverse applications. In this review, various nanozymes that mimic the catalytic activity of different enzymes are introduced. The achievements of nanozymes in different cancer diagnosis and treatment technologies are summarized by highlighting the advantages of nanozymes in these applications. Finally, future research directions in this rapidly developing field are outlooked.
Highlights:
1 This review introduces nanozymes that exhibit different enzymatic activities and emphasizes the advantages of nanozymes over natural enzymes.
2 The roles of nanozymes in different cancer diagnostic and therapeutic technologies are summarized, explained by representative examples.
3 The potential challenges of nanozyme-based cancer theranostics are outlined, and future research directions are outlooked.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram et al., Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021). https://doi.org/10.3322/caac.21660
- E. Tasciotti, X. Liu, R. Bhavane, K. Plant, A.D. Leonard et al., Mesoporous silicon ps as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 3, 151–157 (2008). https://doi.org/10.1038/nnano.2008.34
- T. Lammers, S. Aime, W.E. Hennink, G. Storm, F. Kiessling, Theranostic nanomedicine. Acc. Chem. Res. 44(10), 1029–1038 (2011). https://doi.org/10.1021/ar200019c
- C.E. Ashley, E.C. Carnes, G.K. Phillips, D. Padilla, P.N. Durfee et al., The targeted delivery of multicomponent cargos to cancer cells by nanoporous p-supported lipid bilayers. Nat. Mater. 10, 389–397 (2011). https://doi.org/10.1038/nmat2992
- X. Zhang, X. Chen, Y. Guo, G. Gao, D. Wang et al., Dual gate-controlled therapeutics for overcoming bacterium-induced drug resistance and potentiating cancer immunotherapy. Angew. Chem. Int. Ed. 60(25), 14013–14021 (2021). https://doi.org/10.1002/anie.202102059
- Y. Yuan, J. Zhang, X. Qi, S. Li, G. Liu et al., Furin-mediated intracellular self-assembly of olsalazine nanops for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 18, 1376–1383 (2019). https://doi.org/10.1038/s41563-019-0503-4
- X. Zhang, X. Chen, Y. Guo, H.R. Jia, Y.W. Jiang et al., Endosome/lysosome-detained supramolecular nanogels as an efflux retarder and autophagy inhibitor for repeated photodynamic therapy of multidrug-resistant cancer. Nanoscale Horiz. 5(3), 481–487 (2020). https://doi.org/10.1039/c9nh00643e
- S. Li, W. Su, H. Wu, T. Yuan, C. Yuan et al., Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat. Biomed. Eng. 4, 704–716 (2020). https://doi.org/10.1038/s41551-020-0540-y
- X. Zhang, X. Chen, J. Song, J. Zhang, X. Ren et al., Size-transformable nanostructures: from design to biomedical applications. Adv. Mater. 32(48), 2003752 (2020). https://doi.org/10.1002/adma.202003752
- J. Liu, J. Huang, L. Zhang, J. Lei, Multifunctional metal−organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 50(2), 1188–1218 (2021). https://doi.org/10.1039/d0cs00178c
- S. Yao, Z. Liu, L. Li, Recent progress in nanoscale covalent organic frameworks for cancer diagnosis and therapy. Nano-Micro Lett. 13, 176 (2021). https://doi.org/10.1007/s40820-021-00696-2
- M.M. Mohamed, B.F. Sloane, Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 6, 764–775 (2006). https://doi.org/10.1038/nrc1949
- P.K. Robinson, Enzymes: principles and biotechnological applications. Essays Biochem. 59, 1–41 (2015). https://doi.org/10.1042/bse0590001
- O. Kirk, T.V. Borchert, C.C. Fuglsang, Industrial enzyme applications. Curr. Opin. Biotechnol. 13(4), 345–351 (2002). https://doi.org/10.1016/s0958-1669(02)00328-2
- X. Wang, Y. Hu, H. Wei, Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg. Chem. Front. 3(1), 41–60 (2016). https://doi.org/10.1039/c5qi00240k
- D. Wang, H. Wu, W.Q. Lim, S.Z.F. Phua, P. Xu et al., A mesoporous nanoenzyme derived from metal–organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv. Mater. 31(27), 1901893 (2019). https://doi.org/10.1002/adma.201901893
- D. Jiang, D. Ni, Z.T. Rosenkrans, P. Huang, X. Yan et al., Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
- H. Wei, L. Gao, K. Fan, J. Liu, J. He et al., Nanozymes: a clear definition with fuzzy edges. Nano Today 40, 101269 (2021). https://doi.org/10.1016/j.nantod.2021.101269
- M. Liang, X. Yan, Nanozymes: from new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52(8), 2190–2200 (2019). https://doi.org/10.1021/acs.accounts.9b00140
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li et al., Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48(4), 1004–1076 (2019). https://doi.org/10.1039/c8cs00457a
- Y. Huang, J. Ren, X. Qu, Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119(6), 4357–4412 (2019). https://doi.org/10.1021/acs.chemrev.8b00672
- J. Lou-Franco, B. Das, C. Elliott, C. Cao, Gold nanozymes: from concept to biomedical applications. Nano-Micro Lett. 13, 10 (2020). https://doi.org/10.1007/s40820-020-00532-z
- B. Das, J.L. Franco, N. Logan, P. Balasubramanian, M.I. Kim et al., Nanozymes in point-of-care diagnosis: an emerging futuristic approach for biosensing. Nano-Micro Lett. 13, 193 (2021). https://doi.org/10.1007/s40820-021-00717-0
- Q. Liu, A. Zhang, R. Wang, Q. Zhang, D. Cui, A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett. 13, 154 (2021). https://doi.org/10.1007/s40820-021-00674-8
- X. Liu, Y. Gao, R. Chandrawati, L. Hosta-Rigau, Therapeutic applications of multifunctional nanozymes. Nanoscale 11(44), 21046–21060 (2019). https://doi.org/10.1039/c9nr06596b
- J. Han, J. Yoon, Supramolecular nanozyme-based cancer catalytic therapy. ACS Appl. Bio Mater. 3(11), 7344–7351 (2020). https://doi.org/10.1021/acsabm.0c01127
- M. Li, H. Zhang, Y. Hou, X. Wang, C. Xue et al., State-of-the-art iron-based nanozymes for biocatalytic tumor therapy. Nanoscale Horiz. 5(2), 202–217 (2020). https://doi.org/10.1039/c9nh00577c
- J. Ma, J. Qiu, S. Wang, Nanozymes for catalytic cancer immunotherapy. ACS Appl. Nano Mater. 3(6), 4925–4943 (2020). https://doi.org/10.1021/acsanm.0c00396
- D. Wang, D. Jana, Y. Zhao, Metal−organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 53(7), 1389–1400 (2020). https://doi.org/10.1021/acs.accounts.0c00268
- D. Wang, Y. Zhao, Single-atom engineering of metal−organic frameworks toward healthcare. Chem 7(10), 2635–2671 (2021). https://doi.org/10.1016/j.chempr.2021.08.020
- W. Zhang, J. Liu, X. Li, Y. Zheng, L. Chen et al., Precise chemodynamic therapy of cancer by trifunctional bacterium-based nanozymes. ACS Nano 15(12), 19321–19333 (2021). https://doi.org/10.1021/acsnano.1c05605
- S. Dong, Y. Dong, B. Liu, J. Liu, S. Liu et al., Guiding transition metal-doped hollow cerium tandem nanozymes with elaborately regulated multi-enzymatic activities for intensive chemodynamic therapy. Adv. Mater. 34(7), 2107054 (2022). https://doi.org/10.1002/adma.202107054
- M.I. Kim, Y. Ye, M.A. Woo, J. Lee, H.G. Park, A highly efficient colorimetric immunoassay using a nanocomposite entrapping magnetic and platinum nanops in ordered mesoporous carbon. Adv. Healthcare Mater. 3(1), 36–41 (2014). https://doi.org/10.1002/adhm.201300100
- F. Gong, N. Yang, Y. Wang, M. Zhuo, Q. Zhao et al., Oxygen-deficient bimetallic oxide fewox nanosheets as peroxidase-like nanozyme for sensing cancer via photoacoustic imaging. Small 16(46), 2003496 (2020). https://doi.org/10.1002/smll.202003496
- X. Hu, F. Li, F. Xia, X. Guo, N. Wang et al., Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy. J. Am. Chem. Soc. 142(3), 1636–1644 (2020). https://doi.org/10.1021/jacs.9b13586
- C. Liu, J. Xing, O.U. Akakuru, L. Luo, S. Sun et al., Nanozymes-engineered metal−organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett. 19(8), 5674–5682 (2019). https://doi.org/10.1021/acs.nanolett.9b02253
- D. Jana, D. Wang, A.K. Bindra, Y. Guo, J. Liu et al., Ultrasmall alloy nanozyme for ultrasound- and near-infrared light-promoted tumor ablation. ACS Nano 15(4), 7774–7782 (2021). https://doi.org/10.1021/acsnano.1c01830
- D. Wang, H. Wu, S.Z.F. Phua, G. Yang, W.Q. Lim et al., Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 11, 357 (2020). https://doi.org/10.1038/s41467-019-14199-7
- Z. Wang, X. Zhen, P.K. Upputuri, Y. Jiang, J. Lau et al., Redox-activatable and acid-enhanced for second near-infrared photoacoustic tomography and combined photothermal tumor therapy. ACS Nano 13(5), 5816–5825 (2019). https://doi.org/10.1021/acsnano.9b01411
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang et al., Intrinsic peroxidase-like activity of ferromagnetic nanops. Nat. Nanotech. 2, 577–583 (2007). https://doi.org/10.1038/nnano.2007.260
- C. Lu, X. Liu, Y. Li, F. Yu, L. Tang et al., Multifunctional Janus hematite-silica nanops: mimicking peroxidase-like activity and sensitive colorimetric detection of glucose. ACS Appl. Mater. Interfaces 7(28), 15395–15402 (2015). https://doi.org/10.1021/acsami.5b03423
- X. Huang, F. Xia, Z. Nan, Fabrication of FeS2/SiO2 double mesoporous hollow spheres as an artificial peroxidase and rapid determination of H2O2 and glutathione. ACS Appl. Mater. Interfaces 12(41), 46539–46548 (2020). https://doi.org/10.1021/acsami.0c12593
- J. Chen, Q. Wang, L. Huang, H. Zhang, K. Rong et al., Prussian blue with intrinsic heme-like structure as peroxidase mimic. Nano Res. 11, 4905–4913 (2018). https://doi.org/10.1007/s12274-018-2079-8
- W. Liu, L. Chu, C. Zhang, P. Ni, Y. Jiang et al., Hemin-assisted synthesis of peroxidase-like Fe-N-C nanozymes for detection of ascorbic acid-generating bio-enzymes. Chem. Eng. J. 415, 128876 (2021). https://doi.org/10.1016/j.cej.2021.128876
- R. Geng, R. Chang, Q. Zou, G. Shen, T. Jiao et al., Biomimetic nanozymes based on coassembly of amino acid and hemin for catalytic oxidation and sensing of biomolecules. Small 17(19), 2008114 (2021). https://doi.org/10.1002/smll.202008114
- R. André, F. Natálio, M. Humanes, J. Leppin, K. Heinze et al., V2O5 nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 21(3), 501–509 (2011). https://doi.org/10.1002/adfm.201001302
- S. Sahar, A. Zeb, C. Ling, A. Raja, G. Wang et al., A hybrid VOx incorporated hexacyanoferrate nanostructured hydrogel as a multienzyme mimetic via cascade reactions. ACS Nano 14(3), 3017–3031 (2020). https://doi.org/10.1021/acsnano.9b07886
- A. Sajjad, S.H. Bhatti, Z. Ali, G.H. Jaffari, N.A. Khan et al., Photoinduced fabrication of zinc oxide nanops: transformation of morphological and biological response on light irradiance. ACS Omega 6(17), 11783–11793 (2021). https://doi.org/10.1021/acsomega.1c01512
- S.H. Wen, X.L. Zhong, Y.D. Wu, R.P. Liang, L. Zhang et al., Colorimetric assay conversion to highly sensitive electrochemical assay for bimodal detection of arsenate based on cobalt oxyhydroxide nanozyme via arsenate absorption. Anal. Chem. 91(10), 6487–6497 (2019). https://doi.org/10.1021/acs.analchem.8b05121
- J. Wu, Q. Yang, Q. Li, H. Li, F. Li, Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 93(8), 4084–4091 (2021). https://doi.org/10.1021/acs.analchem.0c05257
- Y. Wang, G. Jia, X. Cui, X. Zhao, Q. Zhang et al., Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specificity. Chem 7(2), 436–449 (2021). https://doi.org/10.1016/j.chempr.2020.10.023
- T.M. Chen, X.J. Wu, J.X. Wang, G.W. Yang, WSe2 few layers with enzyme mimic activity for high-sensitive and high-selective visual detection of glucose. Nanoscale 9(32), 11806–11813 (2017). https://doi.org/10.1039/c7nr03179c
- Y. Liu, N. Nie, H. Tang, C. Zhang, K. Chen et al., Effective antibacterial activity of degradable copper-doped phosphate-based glass nanozymes. ACS Appl. Mater. Interfaces 13(10), 11631–11645 (2021). https://doi.org/10.1021/acsami.0c22746
- B. Navyatha, S. Singh, S. Nara, Auperoxidase nanozymes: promises and applications in biosensing. Biosens. Bioelectron. 175, 112882 (2021). https://doi.org/10.1016/j.bios.2020.112882
- D. Wang, B. Zhang, H. Ding, D. Liu, J. Xiang et al., TiO2 supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today 40, 101243 (2021). https://doi.org/10.1016/j.nantod.2021.101243
- L. Jin, Z. Meng, Y. Zhang, S. Cai, Z. Zhang et al., Ultrasmall Pt nanoclusters as robust peroxidase mimics for colorimetric detection of glucose in human serum. ACS Appl. Mater. Interfaces 9(11), 10027–10033 (2017). https://doi.org/10.1021/acsami.7b01616
- Q. Huang, J. Zhang, W. Li, Y. Fu, A heparin-modified palladium nanozyme for photometric determination of protamine. Microchim. Acta 187, 226 (2020). https://doi.org/10.1007/s00604-020-4208-9
- G. Yim, C.Y. Kim, S. Kang, D.H. Min, K. Kang et al., Intrinsic peroxidase-mimicking Ir nanoplates for nanozymatic anticancer and antibacterial treatment. ACS Appl. Mater. Interfaces 12(37), 41062–41070 (2020). https://doi.org/10.1021/acsami.0c10981
- S. He, L. Yang, P. Balasubramanian, S. Li, H. Peng et al., Osmium nanozyme as peroxidase mimic with high performance and negligible interference of O2. J. Mater. Chem. A 8(47), 25226–25234 (2020). https://doi.org/10.1039/d0ta09247a
- L. He, Y. Li, Q. Wu, D.M. Wang, C.M. Li et al., Ru(III)-based metal−organic gels: Intrinsic horseradish and NADH peroxidase-mimicking nanozyme. ACS Appl. Mater. Interfaces 11(32), 29158–29166 (2019). https://doi.org/10.1021/acsami.9b09283
- S. Ghosh, P. Roy, N. Karmodak, E.D. Jemmis, G. Mugesh, Nanoisozymes: crystal-facet-dependent enzyme-mimetic activity of V2O5 nanomaterials. Angew. Chem. Int. Ed. 57(17), 4510–4515 (2018). https://doi.org/10.1002/anie.201800681
- R. Zhang, X. Yan, K. Fan, Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2(7), 534–547 (2021). https://doi.org/10.1021/accountsmr.1c00074
- H. Wang, P. Li, D. Yu, Y. Zhang, Z. Wang et al., Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 18(6), 3344–3351 (2018). https://doi.org/10.1021/acs.nanolett.7b05095
- Y. Song, X. Wang, C. Zhao, K. Qu, J. Ren et al., Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 16(12), 3617–3621 (2010). https://doi.org/10.1002/chem.200902643
- Y. Song, K. Qu, C. Zhao, J. Ren, X. Qu, Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 22(19), 2206–2210 (2010). https://doi.org/10.1002/adma.200903783
- Q. Zhong, Y. Chen, A. Su, Y. Wang, Synthesis of catalytically active carbon quantum dots and its application for colorimetric detection of glutathione. Sens. Actuators B 273, 1098–1102 (2018). https://doi.org/10.1016/j.snb.2018.07.026
- H. Wang, C. Liu, Z. Liu, J. Ren, X. Qu, Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small 14(13), 1703710 (2018). https://doi.org/10.1002/smll.201703710
- M. Comotti, C.D. Pina, R. Matarrese, M. Rossi, The catalytic activity of “naked” gold ps. Angew. Chem. Int. Ed. 43(43), 5812–5815 (2004). https://doi.org/10.1002/anie.200460446
- C. Liu, Y. Cai, J. Wang, X. Liu, H. Ren et al., Facile preparation of homogeneous copper nanoclusters exhibiting excellent tetraenzyme mimetic activities for colorimetric glutathione sensing and fluorimetric ascorbic acid sensing. ACS Appl. Mater. Interfaces 12(38), 42521–42530 (2020). https://doi.org/10.1021/acsami.0c11983
- M.E. Hafez, H. Ma, W. Ma, Y.T. Long, Unveiling the intrinsic catalytic activities of single-gold-nanop-based enzyme mimetics. Angew. Chem. Int. Ed. 58(19), 6327–6332 (2019). https://doi.org/10.1002/anie.201901384
- W. Luo, C. Zhu, S. Su, D. Li, Y. He et al., Self-catalyzed, self-limiting growth of glucose oxidase-mimicking gold nanops. ACS Nano 4(12), 7451–7458 (2010). https://doi.org/10.1021/nn102592h
- G.L. Wang, L.Y. Jin, X.M. Wu, Y.M. Dong, Z.J. Li, Label-free colorimetric sensor for mercury(II) and DNA on the basis of mercury(II) switched-on the oxidase-mimicking activity of silver nanoclusters. Anal. Chim. Acta 871, 1–8 (2015). https://doi.org/10.1016/j.aca.2015.02.027
- C.J. Yu, T.H. Chen, J.Y. Jiang, W.L. Tseng, Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale 6(16), 9618–9624 (2014). https://doi.org/10.1039/c3nr06896j
- N. Qiu, Y. Liu, R. Guo, Electrodeposition-assisted rapid preparation of Pt nanocluster/3D graphene hybrid nanozymes with outstanding multiple oxidase-like activity for distinguishing colorimetric determination of dihydroxybenzene isomers. ACS Appl. Mater. Interfaces 12(13), 15553–15561 (2020). https://doi.org/10.1021/acsami.9b23546
- M. Cui, Y. Zhao, C. Wang, Q. Song, The oxidase-like activity of iridium nanops, and their application to colorimetric determination of dissolved oxygen. Microchim. Acta 184, 3113–3119 (2017). https://doi.org/10.1007/s00604-017-2326-9
- D. Wang, H. Wu, C. Wang, L. Gu, H. Chen et al., Self-assembled single-site nanozyme for tumor-specific amplified cascade enzymatic therapy. Angew. Chem. Int. Ed. 60(6), 3001–3007 (2021). https://doi.org/10.1002/anie.202008868
- A. Asati, S. Santra, C. Kaittanis, S. Nath, J.M. Perez, Oxidase-like activity of polymer-coated cerium oxide nanops. Angew. Chem. Int. Ed. 48(13), 2308–2312 (2009). https://doi.org/10.1002/anie.200805279
- R. Pautler, E.Y. Kelly, P.J. Huang, J. Cao, B. Liu et al., Attaching DNA to nanoceria: regulating oxidase activity and fluorescence quenching. ACS Appl. Mater. Interfaces 5(15), 6820–6825 (2013). https://doi.org/10.1021/am4018863
- H. Cheng, S. Lin, F. Muhammad, Y.W. Lin, H. Wei, Rationally modulate the oxidase-like activity of nanoceria for self-regulated bioassays. ACS Sens. 1(11), 1336–1343 (2016). https://doi.org/10.1021/acssensors.6b00500
- L. Huang, W. Zhang, K. Chen, W. Zhu, X. Liu et al., Facet-selective response of trigger molecule to CeO2 {1 1 0} for up-regulating oxidase-like activity. Chem. Eng. J. 330, 746–752 (2017). https://doi.org/10.1016/j.cej.2017.08.026
- F. Cao, Y. Zhang, Y. Sun, Z. Wang, L. Zhang et al., Ultrasmall nanozymes isolated within porous carbonaceous frameworks for synergistic cancer therapy: enhanced oxidative damage and reduced energy supply. Chem. Mater. 30(21), 7831–7839 (2018). https://doi.org/10.1021/acs.chemmater.8b03348
- J. Han, K. Liu, R. Chang, L. Zhao, X. Yan, Photooxidase-mimicking nanovesicles with superior photocatalytic activity and stability based on amphiphilic amino acid and phthalocyanine co-assembly. Angew. Chem. Int. Ed. 58(7), 2000–2004 (2019). https://doi.org/10.1002/anie.201811478
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang et al., Nanozyme decorated metal−organic frameworks for enhanced photodynamic therapy. ACS Nano 12(1), 651–661 (2018). https://doi.org/10.1021/acsnano.7b07746
- C. Ren, X. Hu, Q. Zhou, Graphene oxide quantum dots reduce oxidative stress and inhibit neurotoxicity in vitro and in vivo through catalase-like activity and metabolic regulation. Adv. Sci. 5(5), 1700595 (2018). https://doi.org/10.1002/advs.201700595
- S. Li, L. Shang, B. Xu, S. Wang, K. Gu et al., A nanozyme with photo-enhanced dual enzyme-like activities for deep pancreatic cancer therapy. Angew. Chem. Int. Ed. 58(36), 12624–12631 (2019). https://doi.org/10.1002/anie.201904751
- Q. Dan, D. Hu, Y. Ge, S. Zhang, S. Li et al., Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomater. Sci. 8(3), 973–987 (2020). https://doi.org/10.1039/c9bm01742a
- Y. Li, P. Sun, L. Zhao, X. Yan, D.K.P. Ng et al., Ferric ion driven assembly of catalase-like supramolecular photosensitizing nanozymes for combating hypoxic tumors. Angew. Chem. Int. Ed. 59(51), 23228–23238 (2020). https://doi.org/10.1002/anie.202010005
- R. Zhang, L. Chen, Q. Liang, J. Xi, H. Zhao et al., Unveiling the active sites on ferrihydrite with apparent catalase-like activity for potentiating radiotherapy. Nano Today 41, 101317 (2021). https://doi.org/10.1016/j.nantod.2021.101317
- Y. Cheng, C. Cheng, J. Yao, Y. Yu, Y. Liu et al., Mn3O4 nanozyme for inflammatory bowel disease therapy. Adv. Therap. 4(9), 2100081 (2021). https://doi.org/10.1002/adtp.202100081
- C. Wang, Y. Li, W. Yang, L. Zhou, S. Wei, Nanozyme with robust catalase activity by multiple mechanisms and its application for hypoxic tumor treatment. Adv. Healthcare Mater. 10(19), 2100601 (2021). https://doi.org/10.1002/adhm.202100601
- Q. Li, Y. Gao, J. Shen, X. Mu, J. Wang et al., Catalase-like quantum dots of L-lysine polymerization as free radical scavengers for hypoxic brain injury. Mater. Today Commum. 27, 102286 (2021). https://doi.org/10.1016/j.mtcomm.2021.102286
- M. Jiao, Z. Li, X. Li, Z. Zhang, Q. Yuan et al., Solving the H2O2 by-product problem using a catalase-mimicking nanozyme cascade to enhance glycolic acid oxidase. Chem. Eng. J. 388, 124249 (2020). https://doi.org/10.1016/j.cej.2020.124249
- Y. Huang, Z. Liu, C. Liu, E. Ju, Y. Zhang et al., Self-assembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. Int. Ed. 55(23), 6646–6650 (2016). https://doi.org/10.1002/anie.201600868
- J. Mu, X. Zhao, J. Li, E.C. Yang, X.J. Zhao, Novel hierarchical NiO nanoflowers exhibiting intrinsic superoxide dismutase-like activity. J. Mater. Chem. B 4(31), 5217–5221 (2016). https://doi.org/10.1039/c6tb01390b
- W. Zhang, S. Hu, J.J. Yin, W. He, W. Lu et al., Prussian blue nanops as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138(18), 5860–5865 (2016). https://doi.org/10.1021/jacs.5b12070
- K. Korschelt, R. Ragg, C.S. Metzger, M. Kluenker, M. Oster et al., Glycine-functionalized copper(II) hydroxide nanops with high intrinsic superoxide dismutase activity. Nanoscale 9(11), 3952–3960 (2017). https://doi.org/10.1039/c6nr09810j
- N. Singh, M.A. Savanur, S. Srivastava, P. D’Silva, G. Mugesh, A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. Int. Ed. 56(45), 14267–14271 (2017). https://doi.org/10.1002/anie.201708573
- X. Ren, M. Wang, X. He, Z. Li, J. Zhang et al., Superoxide dismutase mimetic ability of Mn-doped ZnS QDs. Chin. Chem. Lett. 29(12), 1865–1868 (2018). https://doi.org/10.1016/j.cclet.2018.12.007
- L. Zhang, Y. Zhang, Z. Wang, F. Cao, Y. Sang et al., Constructing metal–organic framework nanodots as bio-inspired artificial superoxide dismutase for alleviating endotoxemia. Mater. Horiz. 6(8), 1682–1687 (2019). https://doi.org/10.1039/c9mh00339h
- B.C. Yan, J. Cao, J. Liu, Y. Gu, Z. Xu et al., Dietary Fe3O4 nanozymes prevent the injury of neurons and blood-brain barrier integrity from cerebral ischemic stroke. ACS Biomater. Sci. Eng. 7(1), 299–310 (2021). https://doi.org/10.1021/acsbiomaterials.0c01312
- C. Korsvik, S. Patil, S. Seal, W.T. Self, Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanops. Chem. Commun. 10, 1056–1058 (2007). https://doi.org/10.1039/b615134e
- E.L. Samuel, D.C. Marcano, V. Berka, B.R. Bitner, G. Wu et al., Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. PNAS 112(8), 2343–2348 (2015). https://doi.org/10.1073/pnas.1417047112
- W.M. Linehan, G. Bratslavsky, P.A. Pinto, L.S. Schmidt, L. Neckers et al., Molecular diagnosis and therapy of kidney cancer. Annu. Rev. Med. 61, 329–343 (2010). https://doi.org/10.1146/annurev.med.042808.171650
- X. Zhang, F.G. Wu, P. Liu, N. Gu, Z. Chen, Enhanced fluorescence of gold nanoclusters composed of HAuCl4 and histidine by glutathione: glutathione detection and selective cancer cell imaging. Small 10(24), 5170–5177 (2014). https://doi.org/10.1002/smll.201401658
- Y. Liu, M. Zhou, W. Cao, X. Wang, Q. Wang et al., Light-responsive metal−organic framework as an oxidase mimic for cellular glutathione detection. Anal. Chem. 91(13), 8170–8175 (2019). https://doi.org/10.1021/acs.analchem.9b00512
- H.H. Pang, Y.C. Ke, N.S. Li, Y.T. Chen, C.Y. Huang et al., A new lateral flow plasmonic biosensor based on gold-viral biomineralized nanozyme for on-site intracellular glutathione detection to evaluate drug-resistance level. Biosens. Bioelectron. 165, 112325 (2020). https://doi.org/10.1016/j.bios.2020.112325
- Z. Wang, X. Yang, J. Feng, Y. Tang, Y. Jiang et al., Label-free detection of DNA by combining gated mesoporous silica and catalytic signal amplification of platinum nanops. Analyst 139(23), 6088–6091 (2014). https://doi.org/10.1039/c4an01539h
- R. Bhattacharjee, S. Tanaka, S. Moriam, M.K. Masud, J. Lin et al., Porous nanozymes: the peroxidase-mimetic activity of mesoporous iron oxide for the colorimetric and electrochemical detection of global DNA methylation. J. Mater. Chem. B 6(29), 4783–4791 (2018). https://doi.org/10.1039/c8tb01132j
- J. Huang, L. Jiao, W. Xu, Q. Fang, H. Wang et al., Immobilizing enzymes on noble metal hydrogel nanozymes with synergistically enhanced peroxidase activity for ultrasensitive immunoassays by cascade signal amplification. ACS Appl. Mater. Interfaces 13(28), 33383–33391 (2021). https://doi.org/10.1021/acsami.1c09100
- S.K. Maji, A.K. Mandal, K.T. Nguyen, P. Borah, Y. Zhao, Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanop-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 7(18), 9807–9816 (2015). https://doi.org/10.1021/acsami.5b01758
- B. Jiang, L. Yan, J. Zhang, M. Zhou, G. Shi et al., Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 11(10), 9747–9755 (2019). https://doi.org/10.1021/acsami.8b20942
- Z. Wang, Z. Li, Z. Sun, S. Wang, Z. Ali et al., Visualization nanozyme based on tumor microenvironment “unlocking” for intensive combination therapy of breast cancer. Sci. Adv. 6(48), eabc8733 (2020). https://doi.org/10.1126/sciadv.abc8733
- L. Teng, X. Han, Y. Liu, C. Lu, B. Yin et al., Smart nanozyme platform with activity-correlated ratiometric molecular imaging for predicting therapeutic effects. Angew. Chem. Int. Ed. 60(50), 26142–26150 (2021). https://doi.org/10.1002/anie.202110427
- F. Yang, S. Hu, Y. Zhang, X. Cai, Y. Huang et al., A hydrogen peroxide-responsive O2 nanogenerator for ultrasound and magnetic-resonance dual modality imaging. Adv. Mater. 24(38), 5205–5211 (2012). https://doi.org/10.1002/adma.201202367
- L. Feng, B. Liu, R. Xie, D. Wang, C. Qian et al., An ultrasmall SnFe2O4 nanozyme with endogenous oxygen generation and glutathione depletion for synergistic cancer therapy. Adv. Funct. Mater. 31(5), 2006216 (2020). https://doi.org/10.1002/adfm.202006216
- C. Cao, H. Zou, N. Yang, H. Li, Y. Cai et al., Fe3O4/Ag/Bi2MoO6 photoactivatable nanozyme for self-replenishing and sustainable cascaded nanocatalytic cancer therapy. Adv. Mater. 33(52), 2106996 (2021). https://doi.org/10.1002/adma.202106996
- S. Liang, X. Deng, Y. Chang, C. Sun, S. Shao et al., Intelligent hollow Pt-CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 19(6), 4134–4145 (2019). https://doi.org/10.1021/acs.nanolett.9b01595
- X. Zhong, X. Wang, L. Cheng, Y.A. Tang, G. Zhan et al., GSH-depleted PtCu3 nanocages for chemodynamic- enhanced sonodynamic cancer therapy. Adv. Funct. Mater. 30(4), 1907954 (2019). https://doi.org/10.1002/adfm.201907954
- M. Wang, M. Chang, Q. Chen, D. Wang, C. Li et al., Au2Pt-PEG-Ce6 nanoformulation with dual nanozyme activities for synergistic chemodynamic therapy/phototherapy. Biomaterials 252, 120093 (2020). https://doi.org/10.1016/j.biomaterials.2020.120093
- Z. Ma, M.F. Foda, H. Liang, Y. Zhao, H. Han, In situ nanozyme-amplified NIR-II phototheranostics for tumor-specific imaging and therapy. Adv. Funct. Mater. 31(37), 2103765 (2021). https://doi.org/10.1002/adfm.202103765
- J. Wang, S. Gao, X. Wang, H. Zhang, X. Ren et al., Self-assembled manganese phthalocyanine nanops with enhanced peroxidase-like activity for anti-tumor therapy. Nano Res. 15, 2347–2354 (2022). https://doi.org/10.1007/s12274-021-3854-5
- D. Zhu, H. Chen, C. Huang, G. Li, X. Wang et al., H2O2 self-producing single-atom nanozyme hydrogels as light-controlled oxidative stress amplifier for enhanced synergistic therapy by transforming “cold” tumors. Adv. Funct. Mater. 2110268 (2022). https://doi.org/10.1002/adfm.202110268
- M. Huo, L. Wang, Y. Chen, J. Shi, Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 8, 357 (2017). https://doi.org/10.1038/s41467-017-00424-8
- J. Liu, A. Wang, S. Liu, R. Yang, L. Wang et al., A titanium nitride nanozyme for pH-responsive and irradiation-enhanced cascade-catalytic tumor therapy. Angew. Chem. Int. Ed. 60(48), 25328–25338 (2021). https://doi.org/10.1002/anie.202106750
- S. Gao, H. Lin, H. Zhang, H. Yao, Y. Chen et al., Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv. Sci. 6(3), 1801733 (2019). https://doi.org/10.1002/advs.201801733
- X. Meng, D. Li, L. Chen, H. He, Q. Wang et al., High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano 15(3), 5735–5751 (2021). https://doi.org/10.1021/acsnano.1c01248
- Y. Chen, Z.H. Li, J.J. Hu, S.Y. Peng, L. Rong et al., Remote-controlled multi-enzyme system for enhanced tumor therapy via dark/light relay catalysis. Nanoscale Horiz. 5(2), 283–293 (2020). https://doi.org/10.1039/c9nh00583h
- Y. Zhu, W. Wang, J. Cheng, Y. Qu, Y. Dai et al., Stimuli-responsive manganese single-atom nanozyme for tumor therapy via integrated cascade reactions. Angew. Chem. Int. Ed. 60(17), 9480–9488 (2021). https://doi.org/10.1002/anie.202017152
- Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao et al., Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 9, 3334 (2018). https://doi.org/10.1038/s41467-018-05798-x
- K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu et al., In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 9, 1440 (2018). https://doi.org/10.1038/s41467-018-03903-8
- C. Wei, Y. Liu, X. Zhu, X. Chen, Y. Zhou et al., Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer. Biomaterials 238, 119848 (2020). https://doi.org/10.1016/j.biomaterials.2020.119848
- L. Yang, C. Ren, M. Xu, Y. Song, Q. Lu et al., Rod-shape inorganic biomimetic mutual-reinforcing MnO2-Au nanozymes for catalysis-enhanced hypoxic tumor therapy. Nano Res. 13, 2246–2258 (2020). https://doi.org/10.1007/s12274-020-2844-3
- X. Yang, Y. Yang, F. Gao, J.J. Wei, C.G. Qian et al., Biomimetic hybrid nanozymes with self-supplied H+ and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 19(7), 4334–4342 (2019). https://doi.org/10.1021/acs.nanolett.9b00934
- S. Liang, X. Deng, G. Xu, X. Xiao, M. Wang et al., A novel Pt–TiO2 heterostructure with oxygen-deficient layer as bilaterally enhanced sonosensitizer for synergistic chemo-sonodynamic cancer therapy. Adv. Funct. Mater. 30(13), 1908598 (2020). https://doi.org/10.1002/adfm.201908598
- X. Zhou, M. You, F. Wang, Z. Wang, X. Gao et al., Multifunctional graphdiyne-cerium oxide nanozymes facilitate microrna delivery and attenuate tumor hypoxia for highly efficient radiotherapy of esophageal cancer. Adv. Mater. 33(24), 2100556 (2021). https://doi.org/10.1002/adma.202100556
- M. Wang, H. Li, B. Huang, S. Chen, R. Cui et al., An ultra-stable, oxygen-supply nanoprobe emitting in near-infrared-II window to guide and enhance radiotherapy by promoting anti-tumor immunity. Adv. Healthcare Mater. 10(12), 2100090 (2021). https://doi.org/10.1002/adhm.202100090
- L. Feng, Z. Dong, C. Liang, M. Chen, D. Tao et al., Iridium nanocrystals encapsulated liposomes as near-infrared light controllable nanozymes for enhanced cancer radiotherapy. Biomaterials 181, 81–91 (2018). https://doi.org/10.1016/j.biomaterials.2018.07.049
- B. Xu, Y. Cui, W. Wang, S. Li, C. Lyu et al., Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv. Mater. 32(33), 2003563 (2020). https://doi.org/10.1002/adma.202003563
- X. Ling, X. Chen, I.A. Riddell, W. Tao, J. Wang et al., Glutathione-scavenging poly(disulfide amide) nanops for the effective delivery of Pt(IV) prodrugs and reversal of cisplatin resistance. Nano Lett. 18(7), 4618–4625 (2018). https://doi.org/10.1021/acs.nanolett.8b01924
- X. Zhang, X. Chen, Y.W. Jiang, N. Ma, L.Y. Xia et al., Glutathione-depleting gold nanoclusters for enhanced cancer radiotherapy through synergistic external and internal regulations. ACS Appl. Mater. Interfaces 10(13), 10601–10606 (2018). https://doi.org/10.1021/acsami.8b00207
- H. Fan, G. Yan, Z. Zhao, X. Hu, W. Zhang et al., A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew. Chem. Int. Ed. 55(18), 5477–5482 (2016). https://doi.org/10.1002/anie.201510748
- L.S. Lin, J. Song, L. Song, K. Ke, Y. Liu et al., Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed. 57(18), 4902–4906 (2018). https://doi.org/10.1002/anie.201712027
- B. Geng, S. Xu, P. Li, X. Li, F. Fang et al., Platinum crosslinked carbon dot@TiO2-x p-n junctions for relapse-free sonodynamic tumor eradication via high-yield ROS and GSH depletion. Small 18(6), 2103528 (2022). https://doi.org/10.1002/smll.202103528
- W. Wang, X. Zhang, R. Huang, C.M. Hirschbiegel, H. Wang et al., In situ activation of therapeutics through bioorthogonal catalysis. Adv. Drug Deliv. Rev. 176, 113893 (2021). https://doi.org/10.1016/j.addr.2021.113893
- L.K. Folkes, P. Wardman, Oxidative activation of indole-3-acetic acids to cytotoxic species—a potential new role for plant auxins in cancer therapy. Biochem. Pharmacol. 61(2), 129–136 (2001). https://doi.org/10.1016/S0006-2952(00)00498-6
- Q. Liang, J. Xi, X.J. Gao, R. Zhang, Y. Yang et al., A metal-free nanozyme-activated prodrug strategy for targeted tumor catalytic therapy. Nano Today 35, 100935 (2020). https://doi.org/10.1016/j.nantod.2020.100935
- Z. Wang, R. Zhang, X. Yan, K. Fan, Structure and activity of nanozymes: inspirations for de novo design of nanozymes. Mater. Today 41, 81–119 (2020). https://doi.org/10.1016/j.mattod.2020.08.020
- Y. Guan, M. Li, K. Dong, N. Gao, J. Ren et al., Ceria/POMs hybrid nanops as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials 98, 92–102 (2016). https://doi.org/10.1016/j.biomaterials.2016.05.005
- Z. Chen, H. Ji, C. Liu, W. Bing, Z. Wang et al., A multinuclear metal complex based dnase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angew. Chem. Int. Ed. 55(36), 10732–10736 (2016). https://doi.org/10.1002/anie.201605296
- Z. Chen, C. Zhao, E. Ju, H. Ji, J. Ren et al., Design of surface-active artificial enzyme ps to stabilize pickering emulsions for high-performance biphasic biocatalysis. Adv. Mater. 28(8), 1682–1688 (2016). https://doi.org/10.1002/adma.201504557
- H. Xu, M. Liu, X. Huang, Q. Min, J.J. Zhu, Multiplexed quantitative MALDI MS approach for assessing activity and inhibition of protein kinases based on postenrichment dephosphorylation of phosphopeptides by metal–organic framework-templated porous CeO2. Anal. Chem. 90(16), 9859–9867 (2018). https://doi.org/10.1021/acs.analchem.8b01938
- A. Shrivastava, A.A. Khan, M. Khurshid, M.A. Kalam, S.K. Jain et al., Recent developments in L-asparaginase discovery and its potential as anticancer agent. Crit. Rev. Oncol. Hematol. 100, 1–10 (2016). https://doi.org/10.1016/j.critrevonc.2015.01.002
- G. Tang, J. He, J. Liu, X. Yan, K. Fan, Nanozyme for tumor therapy: surface modification matters. Exploration 1(1), 75–89 (2021). https://doi.org/10.1002/exp.20210005
- Z. Ma, L. Wu, K. Han, H. Han, Pt nanozyme for O2 self-sufficient, tumor-specific oxidative damage and drug resistance reversal. Nanoscale Horiz. 4(5), 1124–1131 (2019). https://doi.org/10.1039/c9nh00088g
References
H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram et al., Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021). https://doi.org/10.3322/caac.21660
E. Tasciotti, X. Liu, R. Bhavane, K. Plant, A.D. Leonard et al., Mesoporous silicon ps as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 3, 151–157 (2008). https://doi.org/10.1038/nnano.2008.34
T. Lammers, S. Aime, W.E. Hennink, G. Storm, F. Kiessling, Theranostic nanomedicine. Acc. Chem. Res. 44(10), 1029–1038 (2011). https://doi.org/10.1021/ar200019c
C.E. Ashley, E.C. Carnes, G.K. Phillips, D. Padilla, P.N. Durfee et al., The targeted delivery of multicomponent cargos to cancer cells by nanoporous p-supported lipid bilayers. Nat. Mater. 10, 389–397 (2011). https://doi.org/10.1038/nmat2992
X. Zhang, X. Chen, Y. Guo, G. Gao, D. Wang et al., Dual gate-controlled therapeutics for overcoming bacterium-induced drug resistance and potentiating cancer immunotherapy. Angew. Chem. Int. Ed. 60(25), 14013–14021 (2021). https://doi.org/10.1002/anie.202102059
Y. Yuan, J. Zhang, X. Qi, S. Li, G. Liu et al., Furin-mediated intracellular self-assembly of olsalazine nanops for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 18, 1376–1383 (2019). https://doi.org/10.1038/s41563-019-0503-4
X. Zhang, X. Chen, Y. Guo, H.R. Jia, Y.W. Jiang et al., Endosome/lysosome-detained supramolecular nanogels as an efflux retarder and autophagy inhibitor for repeated photodynamic therapy of multidrug-resistant cancer. Nanoscale Horiz. 5(3), 481–487 (2020). https://doi.org/10.1039/c9nh00643e
S. Li, W. Su, H. Wu, T. Yuan, C. Yuan et al., Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat. Biomed. Eng. 4, 704–716 (2020). https://doi.org/10.1038/s41551-020-0540-y
X. Zhang, X. Chen, J. Song, J. Zhang, X. Ren et al., Size-transformable nanostructures: from design to biomedical applications. Adv. Mater. 32(48), 2003752 (2020). https://doi.org/10.1002/adma.202003752
J. Liu, J. Huang, L. Zhang, J. Lei, Multifunctional metal−organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 50(2), 1188–1218 (2021). https://doi.org/10.1039/d0cs00178c
S. Yao, Z. Liu, L. Li, Recent progress in nanoscale covalent organic frameworks for cancer diagnosis and therapy. Nano-Micro Lett. 13, 176 (2021). https://doi.org/10.1007/s40820-021-00696-2
M.M. Mohamed, B.F. Sloane, Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 6, 764–775 (2006). https://doi.org/10.1038/nrc1949
P.K. Robinson, Enzymes: principles and biotechnological applications. Essays Biochem. 59, 1–41 (2015). https://doi.org/10.1042/bse0590001
O. Kirk, T.V. Borchert, C.C. Fuglsang, Industrial enzyme applications. Curr. Opin. Biotechnol. 13(4), 345–351 (2002). https://doi.org/10.1016/s0958-1669(02)00328-2
X. Wang, Y. Hu, H. Wei, Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg. Chem. Front. 3(1), 41–60 (2016). https://doi.org/10.1039/c5qi00240k
D. Wang, H. Wu, W.Q. Lim, S.Z.F. Phua, P. Xu et al., A mesoporous nanoenzyme derived from metal–organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv. Mater. 31(27), 1901893 (2019). https://doi.org/10.1002/adma.201901893
D. Jiang, D. Ni, Z.T. Rosenkrans, P. Huang, X. Yan et al., Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
H. Wei, L. Gao, K. Fan, J. Liu, J. He et al., Nanozymes: a clear definition with fuzzy edges. Nano Today 40, 101269 (2021). https://doi.org/10.1016/j.nantod.2021.101269
M. Liang, X. Yan, Nanozymes: from new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52(8), 2190–2200 (2019). https://doi.org/10.1021/acs.accounts.9b00140
J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li et al., Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48(4), 1004–1076 (2019). https://doi.org/10.1039/c8cs00457a
Y. Huang, J. Ren, X. Qu, Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119(6), 4357–4412 (2019). https://doi.org/10.1021/acs.chemrev.8b00672
J. Lou-Franco, B. Das, C. Elliott, C. Cao, Gold nanozymes: from concept to biomedical applications. Nano-Micro Lett. 13, 10 (2020). https://doi.org/10.1007/s40820-020-00532-z
B. Das, J.L. Franco, N. Logan, P. Balasubramanian, M.I. Kim et al., Nanozymes in point-of-care diagnosis: an emerging futuristic approach for biosensing. Nano-Micro Lett. 13, 193 (2021). https://doi.org/10.1007/s40820-021-00717-0
Q. Liu, A. Zhang, R. Wang, Q. Zhang, D. Cui, A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett. 13, 154 (2021). https://doi.org/10.1007/s40820-021-00674-8
X. Liu, Y. Gao, R. Chandrawati, L. Hosta-Rigau, Therapeutic applications of multifunctional nanozymes. Nanoscale 11(44), 21046–21060 (2019). https://doi.org/10.1039/c9nr06596b
J. Han, J. Yoon, Supramolecular nanozyme-based cancer catalytic therapy. ACS Appl. Bio Mater. 3(11), 7344–7351 (2020). https://doi.org/10.1021/acsabm.0c01127
M. Li, H. Zhang, Y. Hou, X. Wang, C. Xue et al., State-of-the-art iron-based nanozymes for biocatalytic tumor therapy. Nanoscale Horiz. 5(2), 202–217 (2020). https://doi.org/10.1039/c9nh00577c
J. Ma, J. Qiu, S. Wang, Nanozymes for catalytic cancer immunotherapy. ACS Appl. Nano Mater. 3(6), 4925–4943 (2020). https://doi.org/10.1021/acsanm.0c00396
D. Wang, D. Jana, Y. Zhao, Metal−organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 53(7), 1389–1400 (2020). https://doi.org/10.1021/acs.accounts.0c00268
D. Wang, Y. Zhao, Single-atom engineering of metal−organic frameworks toward healthcare. Chem 7(10), 2635–2671 (2021). https://doi.org/10.1016/j.chempr.2021.08.020
W. Zhang, J. Liu, X. Li, Y. Zheng, L. Chen et al., Precise chemodynamic therapy of cancer by trifunctional bacterium-based nanozymes. ACS Nano 15(12), 19321–19333 (2021). https://doi.org/10.1021/acsnano.1c05605
S. Dong, Y. Dong, B. Liu, J. Liu, S. Liu et al., Guiding transition metal-doped hollow cerium tandem nanozymes with elaborately regulated multi-enzymatic activities for intensive chemodynamic therapy. Adv. Mater. 34(7), 2107054 (2022). https://doi.org/10.1002/adma.202107054
M.I. Kim, Y. Ye, M.A. Woo, J. Lee, H.G. Park, A highly efficient colorimetric immunoassay using a nanocomposite entrapping magnetic and platinum nanops in ordered mesoporous carbon. Adv. Healthcare Mater. 3(1), 36–41 (2014). https://doi.org/10.1002/adhm.201300100
F. Gong, N. Yang, Y. Wang, M. Zhuo, Q. Zhao et al., Oxygen-deficient bimetallic oxide fewox nanosheets as peroxidase-like nanozyme for sensing cancer via photoacoustic imaging. Small 16(46), 2003496 (2020). https://doi.org/10.1002/smll.202003496
X. Hu, F. Li, F. Xia, X. Guo, N. Wang et al., Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy. J. Am. Chem. Soc. 142(3), 1636–1644 (2020). https://doi.org/10.1021/jacs.9b13586
C. Liu, J. Xing, O.U. Akakuru, L. Luo, S. Sun et al., Nanozymes-engineered metal−organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett. 19(8), 5674–5682 (2019). https://doi.org/10.1021/acs.nanolett.9b02253
D. Jana, D. Wang, A.K. Bindra, Y. Guo, J. Liu et al., Ultrasmall alloy nanozyme for ultrasound- and near-infrared light-promoted tumor ablation. ACS Nano 15(4), 7774–7782 (2021). https://doi.org/10.1021/acsnano.1c01830
D. Wang, H. Wu, S.Z.F. Phua, G. Yang, W.Q. Lim et al., Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 11, 357 (2020). https://doi.org/10.1038/s41467-019-14199-7
Z. Wang, X. Zhen, P.K. Upputuri, Y. Jiang, J. Lau et al., Redox-activatable and acid-enhanced for second near-infrared photoacoustic tomography and combined photothermal tumor therapy. ACS Nano 13(5), 5816–5825 (2019). https://doi.org/10.1021/acsnano.9b01411
L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang et al., Intrinsic peroxidase-like activity of ferromagnetic nanops. Nat. Nanotech. 2, 577–583 (2007). https://doi.org/10.1038/nnano.2007.260
C. Lu, X. Liu, Y. Li, F. Yu, L. Tang et al., Multifunctional Janus hematite-silica nanops: mimicking peroxidase-like activity and sensitive colorimetric detection of glucose. ACS Appl. Mater. Interfaces 7(28), 15395–15402 (2015). https://doi.org/10.1021/acsami.5b03423
X. Huang, F. Xia, Z. Nan, Fabrication of FeS2/SiO2 double mesoporous hollow spheres as an artificial peroxidase and rapid determination of H2O2 and glutathione. ACS Appl. Mater. Interfaces 12(41), 46539–46548 (2020). https://doi.org/10.1021/acsami.0c12593
J. Chen, Q. Wang, L. Huang, H. Zhang, K. Rong et al., Prussian blue with intrinsic heme-like structure as peroxidase mimic. Nano Res. 11, 4905–4913 (2018). https://doi.org/10.1007/s12274-018-2079-8
W. Liu, L. Chu, C. Zhang, P. Ni, Y. Jiang et al., Hemin-assisted synthesis of peroxidase-like Fe-N-C nanozymes for detection of ascorbic acid-generating bio-enzymes. Chem. Eng. J. 415, 128876 (2021). https://doi.org/10.1016/j.cej.2021.128876
R. Geng, R. Chang, Q. Zou, G. Shen, T. Jiao et al., Biomimetic nanozymes based on coassembly of amino acid and hemin for catalytic oxidation and sensing of biomolecules. Small 17(19), 2008114 (2021). https://doi.org/10.1002/smll.202008114
R. André, F. Natálio, M. Humanes, J. Leppin, K. Heinze et al., V2O5 nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 21(3), 501–509 (2011). https://doi.org/10.1002/adfm.201001302
S. Sahar, A. Zeb, C. Ling, A. Raja, G. Wang et al., A hybrid VOx incorporated hexacyanoferrate nanostructured hydrogel as a multienzyme mimetic via cascade reactions. ACS Nano 14(3), 3017–3031 (2020). https://doi.org/10.1021/acsnano.9b07886
A. Sajjad, S.H. Bhatti, Z. Ali, G.H. Jaffari, N.A. Khan et al., Photoinduced fabrication of zinc oxide nanops: transformation of morphological and biological response on light irradiance. ACS Omega 6(17), 11783–11793 (2021). https://doi.org/10.1021/acsomega.1c01512
S.H. Wen, X.L. Zhong, Y.D. Wu, R.P. Liang, L. Zhang et al., Colorimetric assay conversion to highly sensitive electrochemical assay for bimodal detection of arsenate based on cobalt oxyhydroxide nanozyme via arsenate absorption. Anal. Chem. 91(10), 6487–6497 (2019). https://doi.org/10.1021/acs.analchem.8b05121
J. Wu, Q. Yang, Q. Li, H. Li, F. Li, Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 93(8), 4084–4091 (2021). https://doi.org/10.1021/acs.analchem.0c05257
Y. Wang, G. Jia, X. Cui, X. Zhao, Q. Zhang et al., Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specificity. Chem 7(2), 436–449 (2021). https://doi.org/10.1016/j.chempr.2020.10.023
T.M. Chen, X.J. Wu, J.X. Wang, G.W. Yang, WSe2 few layers with enzyme mimic activity for high-sensitive and high-selective visual detection of glucose. Nanoscale 9(32), 11806–11813 (2017). https://doi.org/10.1039/c7nr03179c
Y. Liu, N. Nie, H. Tang, C. Zhang, K. Chen et al., Effective antibacterial activity of degradable copper-doped phosphate-based glass nanozymes. ACS Appl. Mater. Interfaces 13(10), 11631–11645 (2021). https://doi.org/10.1021/acsami.0c22746
B. Navyatha, S. Singh, S. Nara, Auperoxidase nanozymes: promises and applications in biosensing. Biosens. Bioelectron. 175, 112882 (2021). https://doi.org/10.1016/j.bios.2020.112882
D. Wang, B. Zhang, H. Ding, D. Liu, J. Xiang et al., TiO2 supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today 40, 101243 (2021). https://doi.org/10.1016/j.nantod.2021.101243
L. Jin, Z. Meng, Y. Zhang, S. Cai, Z. Zhang et al., Ultrasmall Pt nanoclusters as robust peroxidase mimics for colorimetric detection of glucose in human serum. ACS Appl. Mater. Interfaces 9(11), 10027–10033 (2017). https://doi.org/10.1021/acsami.7b01616
Q. Huang, J. Zhang, W. Li, Y. Fu, A heparin-modified palladium nanozyme for photometric determination of protamine. Microchim. Acta 187, 226 (2020). https://doi.org/10.1007/s00604-020-4208-9
G. Yim, C.Y. Kim, S. Kang, D.H. Min, K. Kang et al., Intrinsic peroxidase-mimicking Ir nanoplates for nanozymatic anticancer and antibacterial treatment. ACS Appl. Mater. Interfaces 12(37), 41062–41070 (2020). https://doi.org/10.1021/acsami.0c10981
S. He, L. Yang, P. Balasubramanian, S. Li, H. Peng et al., Osmium nanozyme as peroxidase mimic with high performance and negligible interference of O2. J. Mater. Chem. A 8(47), 25226–25234 (2020). https://doi.org/10.1039/d0ta09247a
L. He, Y. Li, Q. Wu, D.M. Wang, C.M. Li et al., Ru(III)-based metal−organic gels: Intrinsic horseradish and NADH peroxidase-mimicking nanozyme. ACS Appl. Mater. Interfaces 11(32), 29158–29166 (2019). https://doi.org/10.1021/acsami.9b09283
S. Ghosh, P. Roy, N. Karmodak, E.D. Jemmis, G. Mugesh, Nanoisozymes: crystal-facet-dependent enzyme-mimetic activity of V2O5 nanomaterials. Angew. Chem. Int. Ed. 57(17), 4510–4515 (2018). https://doi.org/10.1002/anie.201800681
R. Zhang, X. Yan, K. Fan, Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2(7), 534–547 (2021). https://doi.org/10.1021/accountsmr.1c00074
H. Wang, P. Li, D. Yu, Y. Zhang, Z. Wang et al., Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 18(6), 3344–3351 (2018). https://doi.org/10.1021/acs.nanolett.7b05095
Y. Song, X. Wang, C. Zhao, K. Qu, J. Ren et al., Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 16(12), 3617–3621 (2010). https://doi.org/10.1002/chem.200902643
Y. Song, K. Qu, C. Zhao, J. Ren, X. Qu, Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 22(19), 2206–2210 (2010). https://doi.org/10.1002/adma.200903783
Q. Zhong, Y. Chen, A. Su, Y. Wang, Synthesis of catalytically active carbon quantum dots and its application for colorimetric detection of glutathione. Sens. Actuators B 273, 1098–1102 (2018). https://doi.org/10.1016/j.snb.2018.07.026
H. Wang, C. Liu, Z. Liu, J. Ren, X. Qu, Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small 14(13), 1703710 (2018). https://doi.org/10.1002/smll.201703710
M. Comotti, C.D. Pina, R. Matarrese, M. Rossi, The catalytic activity of “naked” gold ps. Angew. Chem. Int. Ed. 43(43), 5812–5815 (2004). https://doi.org/10.1002/anie.200460446
C. Liu, Y. Cai, J. Wang, X. Liu, H. Ren et al., Facile preparation of homogeneous copper nanoclusters exhibiting excellent tetraenzyme mimetic activities for colorimetric glutathione sensing and fluorimetric ascorbic acid sensing. ACS Appl. Mater. Interfaces 12(38), 42521–42530 (2020). https://doi.org/10.1021/acsami.0c11983
M.E. Hafez, H. Ma, W. Ma, Y.T. Long, Unveiling the intrinsic catalytic activities of single-gold-nanop-based enzyme mimetics. Angew. Chem. Int. Ed. 58(19), 6327–6332 (2019). https://doi.org/10.1002/anie.201901384
W. Luo, C. Zhu, S. Su, D. Li, Y. He et al., Self-catalyzed, self-limiting growth of glucose oxidase-mimicking gold nanops. ACS Nano 4(12), 7451–7458 (2010). https://doi.org/10.1021/nn102592h
G.L. Wang, L.Y. Jin, X.M. Wu, Y.M. Dong, Z.J. Li, Label-free colorimetric sensor for mercury(II) and DNA on the basis of mercury(II) switched-on the oxidase-mimicking activity of silver nanoclusters. Anal. Chim. Acta 871, 1–8 (2015). https://doi.org/10.1016/j.aca.2015.02.027
C.J. Yu, T.H. Chen, J.Y. Jiang, W.L. Tseng, Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale 6(16), 9618–9624 (2014). https://doi.org/10.1039/c3nr06896j
N. Qiu, Y. Liu, R. Guo, Electrodeposition-assisted rapid preparation of Pt nanocluster/3D graphene hybrid nanozymes with outstanding multiple oxidase-like activity for distinguishing colorimetric determination of dihydroxybenzene isomers. ACS Appl. Mater. Interfaces 12(13), 15553–15561 (2020). https://doi.org/10.1021/acsami.9b23546
M. Cui, Y. Zhao, C. Wang, Q. Song, The oxidase-like activity of iridium nanops, and their application to colorimetric determination of dissolved oxygen. Microchim. Acta 184, 3113–3119 (2017). https://doi.org/10.1007/s00604-017-2326-9
D. Wang, H. Wu, C. Wang, L. Gu, H. Chen et al., Self-assembled single-site nanozyme for tumor-specific amplified cascade enzymatic therapy. Angew. Chem. Int. Ed. 60(6), 3001–3007 (2021). https://doi.org/10.1002/anie.202008868
A. Asati, S. Santra, C. Kaittanis, S. Nath, J.M. Perez, Oxidase-like activity of polymer-coated cerium oxide nanops. Angew. Chem. Int. Ed. 48(13), 2308–2312 (2009). https://doi.org/10.1002/anie.200805279
R. Pautler, E.Y. Kelly, P.J. Huang, J. Cao, B. Liu et al., Attaching DNA to nanoceria: regulating oxidase activity and fluorescence quenching. ACS Appl. Mater. Interfaces 5(15), 6820–6825 (2013). https://doi.org/10.1021/am4018863
H. Cheng, S. Lin, F. Muhammad, Y.W. Lin, H. Wei, Rationally modulate the oxidase-like activity of nanoceria for self-regulated bioassays. ACS Sens. 1(11), 1336–1343 (2016). https://doi.org/10.1021/acssensors.6b00500
L. Huang, W. Zhang, K. Chen, W. Zhu, X. Liu et al., Facet-selective response of trigger molecule to CeO2 {1 1 0} for up-regulating oxidase-like activity. Chem. Eng. J. 330, 746–752 (2017). https://doi.org/10.1016/j.cej.2017.08.026
F. Cao, Y. Zhang, Y. Sun, Z. Wang, L. Zhang et al., Ultrasmall nanozymes isolated within porous carbonaceous frameworks for synergistic cancer therapy: enhanced oxidative damage and reduced energy supply. Chem. Mater. 30(21), 7831–7839 (2018). https://doi.org/10.1021/acs.chemmater.8b03348
J. Han, K. Liu, R. Chang, L. Zhao, X. Yan, Photooxidase-mimicking nanovesicles with superior photocatalytic activity and stability based on amphiphilic amino acid and phthalocyanine co-assembly. Angew. Chem. Int. Ed. 58(7), 2000–2004 (2019). https://doi.org/10.1002/anie.201811478
Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang et al., Nanozyme decorated metal−organic frameworks for enhanced photodynamic therapy. ACS Nano 12(1), 651–661 (2018). https://doi.org/10.1021/acsnano.7b07746
C. Ren, X. Hu, Q. Zhou, Graphene oxide quantum dots reduce oxidative stress and inhibit neurotoxicity in vitro and in vivo through catalase-like activity and metabolic regulation. Adv. Sci. 5(5), 1700595 (2018). https://doi.org/10.1002/advs.201700595
S. Li, L. Shang, B. Xu, S. Wang, K. Gu et al., A nanozyme with photo-enhanced dual enzyme-like activities for deep pancreatic cancer therapy. Angew. Chem. Int. Ed. 58(36), 12624–12631 (2019). https://doi.org/10.1002/anie.201904751
Q. Dan, D. Hu, Y. Ge, S. Zhang, S. Li et al., Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomater. Sci. 8(3), 973–987 (2020). https://doi.org/10.1039/c9bm01742a
Y. Li, P. Sun, L. Zhao, X. Yan, D.K.P. Ng et al., Ferric ion driven assembly of catalase-like supramolecular photosensitizing nanozymes for combating hypoxic tumors. Angew. Chem. Int. Ed. 59(51), 23228–23238 (2020). https://doi.org/10.1002/anie.202010005
R. Zhang, L. Chen, Q. Liang, J. Xi, H. Zhao et al., Unveiling the active sites on ferrihydrite with apparent catalase-like activity for potentiating radiotherapy. Nano Today 41, 101317 (2021). https://doi.org/10.1016/j.nantod.2021.101317
Y. Cheng, C. Cheng, J. Yao, Y. Yu, Y. Liu et al., Mn3O4 nanozyme for inflammatory bowel disease therapy. Adv. Therap. 4(9), 2100081 (2021). https://doi.org/10.1002/adtp.202100081
C. Wang, Y. Li, W. Yang, L. Zhou, S. Wei, Nanozyme with robust catalase activity by multiple mechanisms and its application for hypoxic tumor treatment. Adv. Healthcare Mater. 10(19), 2100601 (2021). https://doi.org/10.1002/adhm.202100601
Q. Li, Y. Gao, J. Shen, X. Mu, J. Wang et al., Catalase-like quantum dots of L-lysine polymerization as free radical scavengers for hypoxic brain injury. Mater. Today Commum. 27, 102286 (2021). https://doi.org/10.1016/j.mtcomm.2021.102286
M. Jiao, Z. Li, X. Li, Z. Zhang, Q. Yuan et al., Solving the H2O2 by-product problem using a catalase-mimicking nanozyme cascade to enhance glycolic acid oxidase. Chem. Eng. J. 388, 124249 (2020). https://doi.org/10.1016/j.cej.2020.124249
Y. Huang, Z. Liu, C. Liu, E. Ju, Y. Zhang et al., Self-assembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. Int. Ed. 55(23), 6646–6650 (2016). https://doi.org/10.1002/anie.201600868
J. Mu, X. Zhao, J. Li, E.C. Yang, X.J. Zhao, Novel hierarchical NiO nanoflowers exhibiting intrinsic superoxide dismutase-like activity. J. Mater. Chem. B 4(31), 5217–5221 (2016). https://doi.org/10.1039/c6tb01390b
W. Zhang, S. Hu, J.J. Yin, W. He, W. Lu et al., Prussian blue nanops as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138(18), 5860–5865 (2016). https://doi.org/10.1021/jacs.5b12070
K. Korschelt, R. Ragg, C.S. Metzger, M. Kluenker, M. Oster et al., Glycine-functionalized copper(II) hydroxide nanops with high intrinsic superoxide dismutase activity. Nanoscale 9(11), 3952–3960 (2017). https://doi.org/10.1039/c6nr09810j
N. Singh, M.A. Savanur, S. Srivastava, P. D’Silva, G. Mugesh, A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. Int. Ed. 56(45), 14267–14271 (2017). https://doi.org/10.1002/anie.201708573
X. Ren, M. Wang, X. He, Z. Li, J. Zhang et al., Superoxide dismutase mimetic ability of Mn-doped ZnS QDs. Chin. Chem. Lett. 29(12), 1865–1868 (2018). https://doi.org/10.1016/j.cclet.2018.12.007
L. Zhang, Y. Zhang, Z. Wang, F. Cao, Y. Sang et al., Constructing metal–organic framework nanodots as bio-inspired artificial superoxide dismutase for alleviating endotoxemia. Mater. Horiz. 6(8), 1682–1687 (2019). https://doi.org/10.1039/c9mh00339h
B.C. Yan, J. Cao, J. Liu, Y. Gu, Z. Xu et al., Dietary Fe3O4 nanozymes prevent the injury of neurons and blood-brain barrier integrity from cerebral ischemic stroke. ACS Biomater. Sci. Eng. 7(1), 299–310 (2021). https://doi.org/10.1021/acsbiomaterials.0c01312
C. Korsvik, S. Patil, S. Seal, W.T. Self, Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanops. Chem. Commun. 10, 1056–1058 (2007). https://doi.org/10.1039/b615134e
E.L. Samuel, D.C. Marcano, V. Berka, B.R. Bitner, G. Wu et al., Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. PNAS 112(8), 2343–2348 (2015). https://doi.org/10.1073/pnas.1417047112
W.M. Linehan, G. Bratslavsky, P.A. Pinto, L.S. Schmidt, L. Neckers et al., Molecular diagnosis and therapy of kidney cancer. Annu. Rev. Med. 61, 329–343 (2010). https://doi.org/10.1146/annurev.med.042808.171650
X. Zhang, F.G. Wu, P. Liu, N. Gu, Z. Chen, Enhanced fluorescence of gold nanoclusters composed of HAuCl4 and histidine by glutathione: glutathione detection and selective cancer cell imaging. Small 10(24), 5170–5177 (2014). https://doi.org/10.1002/smll.201401658
Y. Liu, M. Zhou, W. Cao, X. Wang, Q. Wang et al., Light-responsive metal−organic framework as an oxidase mimic for cellular glutathione detection. Anal. Chem. 91(13), 8170–8175 (2019). https://doi.org/10.1021/acs.analchem.9b00512
H.H. Pang, Y.C. Ke, N.S. Li, Y.T. Chen, C.Y. Huang et al., A new lateral flow plasmonic biosensor based on gold-viral biomineralized nanozyme for on-site intracellular glutathione detection to evaluate drug-resistance level. Biosens. Bioelectron. 165, 112325 (2020). https://doi.org/10.1016/j.bios.2020.112325
Z. Wang, X. Yang, J. Feng, Y. Tang, Y. Jiang et al., Label-free detection of DNA by combining gated mesoporous silica and catalytic signal amplification of platinum nanops. Analyst 139(23), 6088–6091 (2014). https://doi.org/10.1039/c4an01539h
R. Bhattacharjee, S. Tanaka, S. Moriam, M.K. Masud, J. Lin et al., Porous nanozymes: the peroxidase-mimetic activity of mesoporous iron oxide for the colorimetric and electrochemical detection of global DNA methylation. J. Mater. Chem. B 6(29), 4783–4791 (2018). https://doi.org/10.1039/c8tb01132j
J. Huang, L. Jiao, W. Xu, Q. Fang, H. Wang et al., Immobilizing enzymes on noble metal hydrogel nanozymes with synergistically enhanced peroxidase activity for ultrasensitive immunoassays by cascade signal amplification. ACS Appl. Mater. Interfaces 13(28), 33383–33391 (2021). https://doi.org/10.1021/acsami.1c09100
S.K. Maji, A.K. Mandal, K.T. Nguyen, P. Borah, Y. Zhao, Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanop-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 7(18), 9807–9816 (2015). https://doi.org/10.1021/acsami.5b01758
B. Jiang, L. Yan, J. Zhang, M. Zhou, G. Shi et al., Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 11(10), 9747–9755 (2019). https://doi.org/10.1021/acsami.8b20942
Z. Wang, Z. Li, Z. Sun, S. Wang, Z. Ali et al., Visualization nanozyme based on tumor microenvironment “unlocking” for intensive combination therapy of breast cancer. Sci. Adv. 6(48), eabc8733 (2020). https://doi.org/10.1126/sciadv.abc8733
L. Teng, X. Han, Y. Liu, C. Lu, B. Yin et al., Smart nanozyme platform with activity-correlated ratiometric molecular imaging for predicting therapeutic effects. Angew. Chem. Int. Ed. 60(50), 26142–26150 (2021). https://doi.org/10.1002/anie.202110427
F. Yang, S. Hu, Y. Zhang, X. Cai, Y. Huang et al., A hydrogen peroxide-responsive O2 nanogenerator for ultrasound and magnetic-resonance dual modality imaging. Adv. Mater. 24(38), 5205–5211 (2012). https://doi.org/10.1002/adma.201202367
L. Feng, B. Liu, R. Xie, D. Wang, C. Qian et al., An ultrasmall SnFe2O4 nanozyme with endogenous oxygen generation and glutathione depletion for synergistic cancer therapy. Adv. Funct. Mater. 31(5), 2006216 (2020). https://doi.org/10.1002/adfm.202006216
C. Cao, H. Zou, N. Yang, H. Li, Y. Cai et al., Fe3O4/Ag/Bi2MoO6 photoactivatable nanozyme for self-replenishing and sustainable cascaded nanocatalytic cancer therapy. Adv. Mater. 33(52), 2106996 (2021). https://doi.org/10.1002/adma.202106996
S. Liang, X. Deng, Y. Chang, C. Sun, S. Shao et al., Intelligent hollow Pt-CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 19(6), 4134–4145 (2019). https://doi.org/10.1021/acs.nanolett.9b01595
X. Zhong, X. Wang, L. Cheng, Y.A. Tang, G. Zhan et al., GSH-depleted PtCu3 nanocages for chemodynamic- enhanced sonodynamic cancer therapy. Adv. Funct. Mater. 30(4), 1907954 (2019). https://doi.org/10.1002/adfm.201907954
M. Wang, M. Chang, Q. Chen, D. Wang, C. Li et al., Au2Pt-PEG-Ce6 nanoformulation with dual nanozyme activities for synergistic chemodynamic therapy/phototherapy. Biomaterials 252, 120093 (2020). https://doi.org/10.1016/j.biomaterials.2020.120093
Z. Ma, M.F. Foda, H. Liang, Y. Zhao, H. Han, In situ nanozyme-amplified NIR-II phototheranostics for tumor-specific imaging and therapy. Adv. Funct. Mater. 31(37), 2103765 (2021). https://doi.org/10.1002/adfm.202103765
J. Wang, S. Gao, X. Wang, H. Zhang, X. Ren et al., Self-assembled manganese phthalocyanine nanops with enhanced peroxidase-like activity for anti-tumor therapy. Nano Res. 15, 2347–2354 (2022). https://doi.org/10.1007/s12274-021-3854-5
D. Zhu, H. Chen, C. Huang, G. Li, X. Wang et al., H2O2 self-producing single-atom nanozyme hydrogels as light-controlled oxidative stress amplifier for enhanced synergistic therapy by transforming “cold” tumors. Adv. Funct. Mater. 2110268 (2022). https://doi.org/10.1002/adfm.202110268
M. Huo, L. Wang, Y. Chen, J. Shi, Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 8, 357 (2017). https://doi.org/10.1038/s41467-017-00424-8
J. Liu, A. Wang, S. Liu, R. Yang, L. Wang et al., A titanium nitride nanozyme for pH-responsive and irradiation-enhanced cascade-catalytic tumor therapy. Angew. Chem. Int. Ed. 60(48), 25328–25338 (2021). https://doi.org/10.1002/anie.202106750
S. Gao, H. Lin, H. Zhang, H. Yao, Y. Chen et al., Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv. Sci. 6(3), 1801733 (2019). https://doi.org/10.1002/advs.201801733
X. Meng, D. Li, L. Chen, H. He, Q. Wang et al., High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano 15(3), 5735–5751 (2021). https://doi.org/10.1021/acsnano.1c01248
Y. Chen, Z.H. Li, J.J. Hu, S.Y. Peng, L. Rong et al., Remote-controlled multi-enzyme system for enhanced tumor therapy via dark/light relay catalysis. Nanoscale Horiz. 5(2), 283–293 (2020). https://doi.org/10.1039/c9nh00583h
Y. Zhu, W. Wang, J. Cheng, Y. Qu, Y. Dai et al., Stimuli-responsive manganese single-atom nanozyme for tumor therapy via integrated cascade reactions. Angew. Chem. Int. Ed. 60(17), 9480–9488 (2021). https://doi.org/10.1002/anie.202017152
Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao et al., Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 9, 3334 (2018). https://doi.org/10.1038/s41467-018-05798-x
K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu et al., In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 9, 1440 (2018). https://doi.org/10.1038/s41467-018-03903-8
C. Wei, Y. Liu, X. Zhu, X. Chen, Y. Zhou et al., Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer. Biomaterials 238, 119848 (2020). https://doi.org/10.1016/j.biomaterials.2020.119848
L. Yang, C. Ren, M. Xu, Y. Song, Q. Lu et al., Rod-shape inorganic biomimetic mutual-reinforcing MnO2-Au nanozymes for catalysis-enhanced hypoxic tumor therapy. Nano Res. 13, 2246–2258 (2020). https://doi.org/10.1007/s12274-020-2844-3
X. Yang, Y. Yang, F. Gao, J.J. Wei, C.G. Qian et al., Biomimetic hybrid nanozymes with self-supplied H+ and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 19(7), 4334–4342 (2019). https://doi.org/10.1021/acs.nanolett.9b00934
S. Liang, X. Deng, G. Xu, X. Xiao, M. Wang et al., A novel Pt–TiO2 heterostructure with oxygen-deficient layer as bilaterally enhanced sonosensitizer for synergistic chemo-sonodynamic cancer therapy. Adv. Funct. Mater. 30(13), 1908598 (2020). https://doi.org/10.1002/adfm.201908598
X. Zhou, M. You, F. Wang, Z. Wang, X. Gao et al., Multifunctional graphdiyne-cerium oxide nanozymes facilitate microrna delivery and attenuate tumor hypoxia for highly efficient radiotherapy of esophageal cancer. Adv. Mater. 33(24), 2100556 (2021). https://doi.org/10.1002/adma.202100556
M. Wang, H. Li, B. Huang, S. Chen, R. Cui et al., An ultra-stable, oxygen-supply nanoprobe emitting in near-infrared-II window to guide and enhance radiotherapy by promoting anti-tumor immunity. Adv. Healthcare Mater. 10(12), 2100090 (2021). https://doi.org/10.1002/adhm.202100090
L. Feng, Z. Dong, C. Liang, M. Chen, D. Tao et al., Iridium nanocrystals encapsulated liposomes as near-infrared light controllable nanozymes for enhanced cancer radiotherapy. Biomaterials 181, 81–91 (2018). https://doi.org/10.1016/j.biomaterials.2018.07.049
B. Xu, Y. Cui, W. Wang, S. Li, C. Lyu et al., Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv. Mater. 32(33), 2003563 (2020). https://doi.org/10.1002/adma.202003563
X. Ling, X. Chen, I.A. Riddell, W. Tao, J. Wang et al., Glutathione-scavenging poly(disulfide amide) nanops for the effective delivery of Pt(IV) prodrugs and reversal of cisplatin resistance. Nano Lett. 18(7), 4618–4625 (2018). https://doi.org/10.1021/acs.nanolett.8b01924
X. Zhang, X. Chen, Y.W. Jiang, N. Ma, L.Y. Xia et al., Glutathione-depleting gold nanoclusters for enhanced cancer radiotherapy through synergistic external and internal regulations. ACS Appl. Mater. Interfaces 10(13), 10601–10606 (2018). https://doi.org/10.1021/acsami.8b00207
H. Fan, G. Yan, Z. Zhao, X. Hu, W. Zhang et al., A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew. Chem. Int. Ed. 55(18), 5477–5482 (2016). https://doi.org/10.1002/anie.201510748
L.S. Lin, J. Song, L. Song, K. Ke, Y. Liu et al., Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed. 57(18), 4902–4906 (2018). https://doi.org/10.1002/anie.201712027
B. Geng, S. Xu, P. Li, X. Li, F. Fang et al., Platinum crosslinked carbon dot@TiO2-x p-n junctions for relapse-free sonodynamic tumor eradication via high-yield ROS and GSH depletion. Small 18(6), 2103528 (2022). https://doi.org/10.1002/smll.202103528
W. Wang, X. Zhang, R. Huang, C.M. Hirschbiegel, H. Wang et al., In situ activation of therapeutics through bioorthogonal catalysis. Adv. Drug Deliv. Rev. 176, 113893 (2021). https://doi.org/10.1016/j.addr.2021.113893
L.K. Folkes, P. Wardman, Oxidative activation of indole-3-acetic acids to cytotoxic species—a potential new role for plant auxins in cancer therapy. Biochem. Pharmacol. 61(2), 129–136 (2001). https://doi.org/10.1016/S0006-2952(00)00498-6
Q. Liang, J. Xi, X.J. Gao, R. Zhang, Y. Yang et al., A metal-free nanozyme-activated prodrug strategy for targeted tumor catalytic therapy. Nano Today 35, 100935 (2020). https://doi.org/10.1016/j.nantod.2020.100935
Z. Wang, R. Zhang, X. Yan, K. Fan, Structure and activity of nanozymes: inspirations for de novo design of nanozymes. Mater. Today 41, 81–119 (2020). https://doi.org/10.1016/j.mattod.2020.08.020
Y. Guan, M. Li, K. Dong, N. Gao, J. Ren et al., Ceria/POMs hybrid nanops as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials 98, 92–102 (2016). https://doi.org/10.1016/j.biomaterials.2016.05.005
Z. Chen, H. Ji, C. Liu, W. Bing, Z. Wang et al., A multinuclear metal complex based dnase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angew. Chem. Int. Ed. 55(36), 10732–10736 (2016). https://doi.org/10.1002/anie.201605296
Z. Chen, C. Zhao, E. Ju, H. Ji, J. Ren et al., Design of surface-active artificial enzyme ps to stabilize pickering emulsions for high-performance biphasic biocatalysis. Adv. Mater. 28(8), 1682–1688 (2016). https://doi.org/10.1002/adma.201504557
H. Xu, M. Liu, X. Huang, Q. Min, J.J. Zhu, Multiplexed quantitative MALDI MS approach for assessing activity and inhibition of protein kinases based on postenrichment dephosphorylation of phosphopeptides by metal–organic framework-templated porous CeO2. Anal. Chem. 90(16), 9859–9867 (2018). https://doi.org/10.1021/acs.analchem.8b01938
A. Shrivastava, A.A. Khan, M. Khurshid, M.A. Kalam, S.K. Jain et al., Recent developments in L-asparaginase discovery and its potential as anticancer agent. Crit. Rev. Oncol. Hematol. 100, 1–10 (2016). https://doi.org/10.1016/j.critrevonc.2015.01.002
G. Tang, J. He, J. Liu, X. Yan, K. Fan, Nanozyme for tumor therapy: surface modification matters. Exploration 1(1), 75–89 (2021). https://doi.org/10.1002/exp.20210005
Z. Ma, L. Wu, K. Han, H. Han, Pt nanozyme for O2 self-sufficient, tumor-specific oxidative damage and drug resistance reversal. Nanoscale Horiz. 4(5), 1124–1131 (2019). https://doi.org/10.1039/c9nh00088g